Supplemental Digital Content is Available in the Text.
Key Words: left ventricular thrombus, vitamin K antagonists, direct oral anticoagulants
Abstract:
Left ventricular thrombi (LVTs) increase the risk of stroke, systemic embolism, and subsequent death. Current guidelines recommend vitamin K antagonists (VKAs) as first-line treatment for LVT. Direct oral anticoagulants (DOACs) are increasingly used as alternatives to warfarin for the treatment of LVT. However, the efficacy and safety of DOACs versus VKAs remain controversial. Thus, we conducted an updated meta-analysis of DOACs versus VKAs for LVT treatment. We systematically searched PubMed, Embase, ClinicalTrials, and Cochrane Library databases for relevant articles published before December 11, 2021. The relative risks (RRs) with 95% confidence intervals (CIs) were calculated for each study. The meta-analysis included 12 cohort studies and 3 randomized controlled trials with a total of 2334 patients. We found that DOACs had a lower risk of clinically significant bleeding than VKAs (RR = 0.6; 95% CI, 0.39 to 0.90; P = 0.01; I2 = 0%). There was no difference in LVT resolution (RR = 1.01; 95% CI, 0.93 to 1.09; P = 0.48; I2 = 0%), stroke and/or systematic embolic events (RR = 0.87; 95% CI, 0.11 to 1.55; P = 0.2; I2 = 30%), and all-cause mortality (RR = 0.9; 95% CI, 0.58 to 1.4; P = 0.65; I2 = 0%). Overall, DOACs are noninferior to warfarin in LVT treatment but have a lower risk of clinically significant bleeding. This suggests that DOACs might be better alternatives to warfarin for LVT treatment.
INTRODUCTION
Left ventricular thrombus (LVT) is a common complication of acute MI, and it also occurs in patients with nonischemic cardiomyopathy and severe cardiac dysfunction, such as dilated cardiomyopathy, stress cardiomyopathy, and left ventricular noncompaction. Previous studies have shown that LVT significantly increases the risk of stroke, systemic embolism, and subsequent death.1 Current guidelines recommend vitamin K antagonists (VKAs) as the first-line anticoagulant therapy for LVT.2,3 However, VKAs have many disadvantages, such as a slow-onset anticoagulant effect, need for bridging therapy, interaction with drugs and food, individual genetic differences, and need for frequent monitoring of international standardized ratios.
Both controlled clinical trials and real-world studies have shown that direct oral anticoagulants (DOACs) are noninferior to VKAs and have several advantages. Therefore, DOACs are preferred in patients with nonvalvular atrial fibrillation (AF) and venous thromboembolism. Owing to their efficacy and safety, DOACs are increasingly being used as off-label alternatives to VKAs for the treatment of LVT in clinical practice. However, most studies on DOACs for LVT are retrospective cohort studies or case series. Moreover, the conclusions of meta-analyses comparing the efficacy and safety of DOACs and VKAs for LVT treatment are controversial.4,5 Recently, several cohort studies and 3 randomized controlled trials (RCTs) have been completed.6–13 Therefore, we conducted an updated systematic review and meta-analysis to compare the safety and efficacy of DOACs and VKAs in patients with LVTs.
METHOD
We performed an updated systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis.14 The study protocol was registered at www.crd.york.ac.uk/prospero as CRD42021290293.
Search Strategy and Study Selection
We systematically searched PubMed, Embase, ClinicalTrials, and Cochrane Library databases for relevant articles published before December 11, 2021. The following keywords were included “left ventricular thromb*”, “DOAC*”, “NOAC*”, “direct anticoagulant*”, “new oral anticoagulant*”, “warfarin”,“rivaroxaban”, “apixaban”, “edoxaban”, “dabigatran”, and “vitamin K antagonist*”. Articles meeting the following criteria were selected: (1) cohort studies and/or RCTs; (2) adult patients with LVT evaluated using transthoracic echocardiography (TTE), left ventriculography, or cardiac magnetic resonance imaging; (3) comparisons between DOACs and VKAs; and (4) evaluation of safety and efficacy in thromboembolic events, clinical bleeding complications, thrombus resolution, and all-cause mortality. Posters, conference abstracts, and non-English articles were also excluded. Clinical trials with results that met the inclusion criteria were included in this meta-analysis. Any disagreements in the study selection process were resolved by consensus.
Data Extraction and Quality Evaluation
Information about patient characteristics, publication year, study design, study period, sample size, type of VKA, type of DOAC, follow-up duration, and outcome measures was systematically extracted by KW and MZ. The quality of the included cohort study was evaluated using the Newcastle–Ottawa Scale (NOS).15 According to the NOS, selection score for each study was 0–4, comparability score for the study groups was 0–2, and ascertainment score for the outcome of interest was 0–3. The included studies were classified as high (0–4), medium (5–7), or low (8–9) risk of bias. The quality of RCTs was assessed using a modified Jadad 7-point scale, which contains 2 questions each on randomization, concealment of allocation, and double-blinding, and 1 question on the reporting of dropouts and withdrawals.16 A Jadad score of ≥4 was considered high quality and ≤3 was considered low quality. Any disagreement in the data extraction and appraisal process was resolved by a third reviewer (Q.X.).
Outcome Assessment
The effective end points included thromboembolic events (defined as stroke and/or systemic embolic events during the observation period) and thrombus resolution (defined as the absence of thrombus on repeat imaging). Safety end points included clinically significant bleeding and all-cause mortality. Clinically significant bleeding was defined as bleeding requiring clinical management and/or hospitalization as defined in each study.
Statistical Analysis
The relative risks (RRs) with 95% confidence intervals (CIs) were calculated for each study. Statistical heterogeneity was assessed using the I2 test (I2 > 50%, indicated heterogeneity) and χ2 test (P < 0.10, considered significant). We used a random effects model when there was significant heterogeneity among the included trials; otherwise, we used a fixed effects model. We also performed a subgroup analysis to assess the effect of study design (observational study vs. RCTs) on safety and efficacy outcomes. Sensitivity analysis was conducted by “one-study removed” method to examine the credibility of the results. Publication bias was visually assessed using a funnel plot. Egger's linear regression test was performed when the outcomes included 10 or more studies. All analyses were performed using the Review Manager (version 5.4) and R software (version 4.0.3).
RESULTS
Identification and Selection of Studies
After removing duplicates and animal studies, the initial search yielded 163 relevant studies. After screening titles and abstracts, 26 potentially relevant articles were selected and retrieved for more detailed information. Further screening for eligibility was performed by 2 independent reviewers (K.W. and M.Z.), according to the inclusion and exclusion criteria. Ultimately, 12 cohort studies6–10,17–23 and 3 RCTs11–13 met the inclusion criteria and were included in our systematic review (Fig. 1).
FIGURE 1.
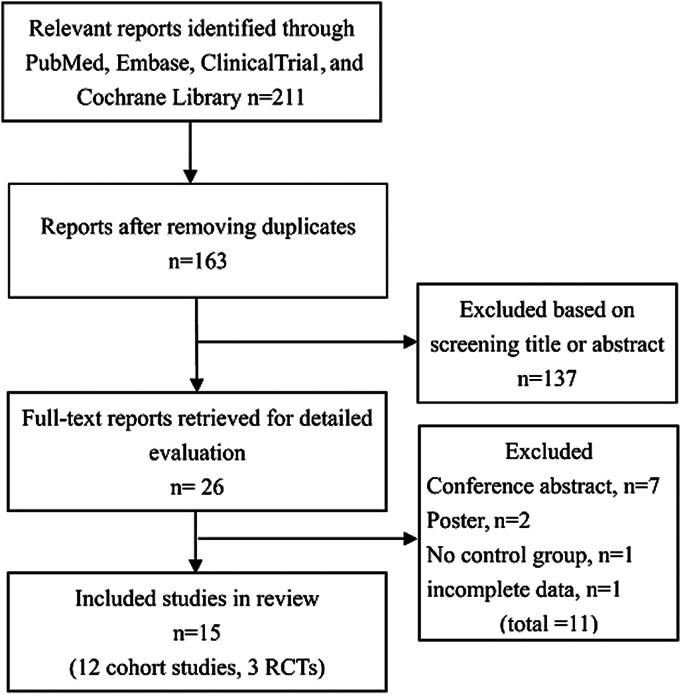
Selection process for studies included in meta-analysis.
A total of 2334 patients were enrolled in the updated meta-analysis; specifically, 1705 patients in the VKA group and 629 patients in the DOAC group. The mean age of the patients with LVT was 60 years; most of the patients were male (74.7%), and 64.8% of patients had ischemic heart disease. TTE was the main diagnostic method for LVT, and cardiac magnetic resonance imaging and left ventricular angiography were used in some patients. All the clinical study results were published between 2020 and 2021. The follow-up duration ranged from 3 months to 36 months. Of the 629 DOACs patients, rivaroxaban (251 of 499, 50.3%) was the most frequently used DOACs, followed by apixaban (202 of 499, 40.4%), dabigatran (44 of 499, 8.8%), and edoxaban (2 of 499, 0.4%); types of DOACs were not reported in the other 130 patients. In most studies, VKAs mainly referred to warfarin (98.4%), with the exception of 1 study in which acenocoumarol and fluindione were also used. Supplemental Digital Content 1 (see Table 1, http://links.lww.com/JCVP/A802) shows the characteristics of the included cohort studies and RCTs. The overall score of the 12 observational cohort studies ranged from 5 to 8 according to the NOS, and the 3 RCTs had an overall score of 3–5 according to the modified Jadad score. The details of the quality assessments are shown in Supplemental Digital Contents 2 and 3 (see Tables 2 and 3, http://links.lww.com/JCVP/A803 and http://links.lww.com/JCVP/A804).
Effective End Points
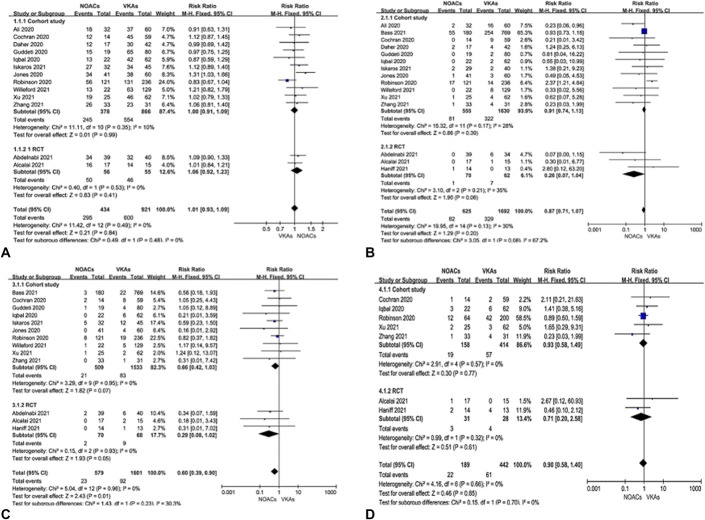
There was no difference between DOACs and VKAs regarding LVT resolution when data from 13 studies7–12,17–23 were pooled (RR = 1.01; 95% CI, 0.93 to 1.09, P = 0.48, I2 = 0%). Similar results were obtained by subgroup analysis based on different study designs (Fig. 2A). Fifteen articles6–13,17–23 reported on stroke and/or systematic embolic events. The pooled RR for stroke and/or systematic embolic events was 0.87 (95% CI, 0.11 to 1.55, P = 0.2), but this analysis demonstrated substantial heterogeneity (I2 = 67.2%) (Fig. 2B). Further subgroup analysis eliminated heterogeneity, and we found that compared with VKAs, the DOAC group in RCTs had a lower thromboembolic event rate (1.4% vs. 11.2%, P = 0.06).
FIGURE 2.
Pooled effects of DOACs versus VKAs on thrombus resolution (A), thromboembolic events (B), significant bleedings (C), and all-cause mortality (D).
Safety End Points
Bleeding events, particularly clinically significant bleeding events, are the focus of anticoagulant safety in LVTs. Thirteen studies (10 cohort studies and 3 RCTs, 2295 patients)6–13,17,20–23 reported bleeding outcomes. Pooling all data, we found that DOACs had a lower risk of clinically significant bleeding than VKAs during LVT treatment (RR = 0.6, 95% CI, 0.39 to 0.90, P = 0.01, I2 = 0%). Further subgroup analysis revealed that, whether in RCTs or cohort studies, the risk of clinical bleeding in the DOAC group was lower than that in the VKA group (RR = 0.29, 95% CI, 0.08 to 1.02, P = 0.05, I2 = 0%; RR = 0.66, 95% CI, 0.42 to 1.03, P = 0.07, I2 = 0%) (Fig. 2C). Regarding all-cause mortality, 7 studies (5 cohort studies and 2 RCTs, 714 patients)9,10,12,13,17,21,23 were included in the meta-analysis. No significant difference was detected between DOACs and VKAs in LVT treatment (RR = 0.9, 95% CI, 0.58 to 1.4, P = 0.65, I2 = 0%) (Fig. 2D).
Publication Bias
Possible publication bias for each end point was assessed using a funnel plot. Except for thromboembolic events, the funnel plot was symmetrical (see Figure 1, Supplemental Digital Content 4, http://links.lww.com/JCVP/A800), and no asymmetry was found when tested using the Egger's test (P for thrombus resolution = 0.7422; P for thromboembolic events = 0.1211; P for clinically significant bleeding = 0.1185). Because only 7 studies were included for all-cause mortality, funnel plots and Egger's test were not performed to test for publication bias.
Sensitivity Analysis
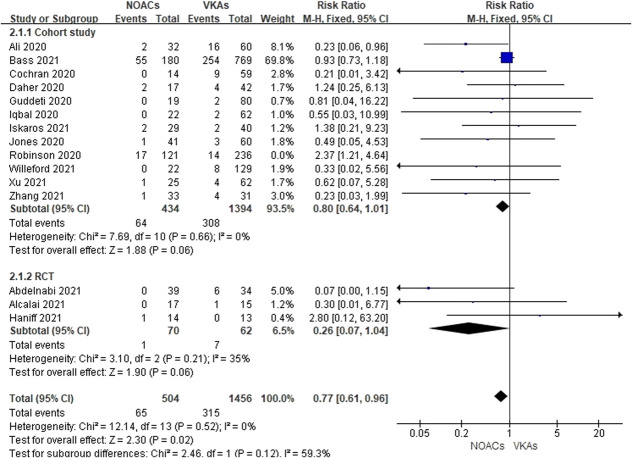
Sensitivity analysis by “one-study removed” method yielded consistent results expect for pooling the data of thromboembolic events (see Figure 2, Supplemental Digital Content 5, http://links.lww.com/JCVP/A801). When Robinson's study was excluded, DOACs showed a trend toward lower thromboembolic events (RR = 0.77, 95% CI, 0.61 to 0.96, P = 0.02, I2 = 0%) (Fig. 3).
FIGURE 3.
Effects of DOACs versus VKAs on thromboembolic events when excluding the study by Robinson.
DISCUSSION
To the best of our knowledge, this is the largest meta-analysis to compare DOACs and VKAs in LVT treatment, which involved 12 cohort studies and 3 RCTs. In this updated meta-analysis, we found that DOACs had a lower risk of clinically significant bleeding than VKAs in LVT treatment. No differences were observed in the LVT resolution, thromboembolic events, or all-cause mortality.
LVT is a common complication of ischemic and nonischemic cardiomyopathies and is associated with systemic thromboembolism and death. In the thrombolytic era (TTE as a diagnostic tool), the incidence of LVT is approximately 17% in all patients after acute MI and up to 34%–57% in those with anterior MI.24 In the percutaneous coronary intervention (PCI) era, the incidence of LVT (assessed by TTE) dropped below 10% owing to the deployment of primary PCI, use of neurohormonal antagonists, and potent antithrombotic combinations.25 However, in patients with anterior MI and left ventricular ejection fraction <50%, the incidence of LVT (assessed by cardiac magnetic resonance imaging) still reached 20%.26,27 Moreover, even in the contemporary PCI era, the incidence of systemic embolisms in patients with LVT remains high. A 2018 study by Maniwa et al28 indicated systemic embolism rates as high as 16.3% in patients with LVT, 5 times higher than those in non-LVT patients. In addition, more than 10% of patients with LVT die within 1 year.29 According to current European and American guidelines, patients with LVT should receive anticoagulant therapy for 3–6 months.2,3 VKAs (mainly warfarin) are recommended as the first-line oral anticoagulants for LVT treatment. Warfarin inhibits the synthesis of coagulation factors IIa, VIIa, IXa, and Xa. Moreover, it also inhibits the synthesis of protein C and protein S. Owing to the delay in factor II inhibition, concomitant administration of unfractionated heparin or low-molecular-weight heparins is required for at least 3 days. Furthermore, warfarin has many other drawbacks, such as drug and food interactions, substantial individual variability in dose response, and frequent monitoring, all of which lead to poor compliance.
In the past 10 years, new DOACs have been approved for anticoagulant treatment of nonvalvular AF and venous thromboembolic diseases.30 The anticoagulation effects of DOACs are achieved by inhibiting thrombin (dabigatran) or factor Ⅹa (rivaroxaban, apixaban, and edoxaban) in the coagulation cascade. DOACs have many attractive properties such as less interaction with food and drugs, less frequent blood tests, and faster onset without the need for bridging therapy. In addition, DOACs can achieve consistent anticoagulant effects. At present, both clinical trials and real-world data confirm that DOACs are superior to warfarin in preventing and treating thromboembolic events in patients with nonvalvular AF.31 Because the pathophysiological mechanism of LVT is similar to that of AF-related thrombi (eg, low flow and low shear environment), NOACs may be applicable for the treatment of LVT. For these reasons, the off-label application of NOACs for the treatment of LVT is becoming popular.
However, the efficacy and safety of DOACs versus warfarin for treating LVT remain controversial.4,5 Recently, Robinson et al23 conducted a multicenter retrospective study including 514 patients with LVT, of whom 186 were treated with apixaban, 300 with warfarin, and 64 with treatment switching. The study showed that apixaban had a higher risk of stroke or systemic embolic events than warfarin did. However, this difference disappeared when the apixaban-only (236 patients) and warfarin-only (121 patients) groups were compared (RR for apixaban vs. warfarin =1.99, 95% CI, 0.91 to 4.35, P = 0.08). The results remained statistically insignificant after intention-to-treat analysis (RR for apixaban vs. warfarin = 1.42, 95% CI, 0.68 to 2.96, P = 0.35). The retrospective nature, lack of randomization, and confounding factors may explain the different conclusions of the study by Robinson et al. This updated meta-analysis showed that there was no difference between DOACs and VKAs regarding stroke and/or systemic embolism when data from 15 studies (12 cohort studies and 3 RCTs, 2334 patients) were pooled. Interestingly, the pooled analysis of the 3 RCTs also showed that DOACs had a lower incidence of thromboembolic events than VKAs (1.4% vs. 11.2%, P = 0.06). However, the follow-up duration for all the 3 RCTS was only 3–6 months. Given that the study by Maniwa28 showed that 53% of thromboembolic events occurred after 6 months, large-scale RCTs with long-term follow-up durations are needed to provide more convincing results. Pooling all the current data, we found that NOACs did not increase the incidence of thromboembolism compared with warfarin.
Clinically significant bleeding is a major concern during LVT treatment. This meta-analysis found that 64.8% of the patients with LVT who had ischemic heart disease required concomitant single or dual antiplatelet therapy. Pooling all 13 studies, we found that DOACs reduced clinically significant bleeding compared with VKAs. Rivaroxaban, dabigatran, and apixaban plus P2Y12 (and/or aspirin) resulted in a lower incidence of major bleeding than conventional warfarin regimens in patients with nonvalvular AF with MI or PCI, which has been confirmed by large RCTs of PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS.32–34 Edoxaban-based regimens had a similar risk of major or clinically relevant nonmajor bleeding as warfarin-based antithrombotic regimens (ENTRUST-AF-PCI).35 In this meta-analysis, the application proportion of rivaroxaban, apixaban, and dabigatran accounted for 99.6%, whereas that of edoxaban was only 0.4%, which led to a similar conclusion that clinically significant bleeding from DOACs was lower than that from VKAs.
Although DOACs have been widely used in recent years, warfarin is still irreplaceable in specific conditions.36 The first condition is anticoagulant therapy for LVT complicated with renal insufficiency. Each DOAC available is excreted through the kidney, and renal dysfunction affects the bioavailability of drugs. Therefore, renal function needs to be evaluated before using DOACs. When creatinine clearance is <15 mL/min, only VKAs can be used. The second condition is anticoagulant treatment of LVT with mechanical prostheses or moderate-to-severe mitral stenosis of rheumatic origin. Given that all phase III clinical trials is comparing DOACs and VKAs exclude DOACs, VKAs are the only oral anticoagulant that can be used in such contexts where DOACs are contraindicated. In short, anticoagulant therapy of LVT is very challenging, taking into account renal function, concomitant disease, and so on. Currently, 2 RCTs (NCT03764241 and NCT04970576) and 1 observational study (NCT05028777) are being conducted to compare the efficacy and safety of DOACs and VKAs in LVT treatment. These results provide further evidence for clinical practice.
Limitations
Our meta-analysis had several limitations. (1) Most of the included studies were retrospective cohort studies, and the sample size of the RCTs was small. (2) Patient characteristics, type of DOACs, time in the therapeutic range of warfarin, antithrombotic regimens, definition of clinical events, and follow-up durations varied between studies. (3) In most studies, LVT was assessed using TTE, which has limited sensitivity, suggesting that LVT may be missed. Therefore, the generalizability of the findings is limited. Larger RCTs with long-term follow-up are expected to provide more convincing evidence to improve generalizability.
CONCLUSIONS
DOACs are noninferior to warfarin in the treatment of LVT but have a lower risk of clinically significant bleeding. This suggests that DOACs might be better alternatives to warfarin for LVT treatment.
Supplementary Material
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jcvp.org).
Y. Chen, M. Zhu, and K. Wang have contributed equally to this work.
J. Ma and Q. Xu were involved in the design of the study and critical revision of the manuscript, supervised the process, and arbitrated when necessary. Y. Chen and M. Zhu were involved in quality assessment of the studies, data extraction and analysis, and manuscript writing. Y. Chen and K. Wang were involved in the data analysis, interpretation of data, and critical revision of the manuscript. All authors have approved the manuscript for publication.
REFERENCES
- 1.Lattuca B, Bouziri N, Kerneis M, et al. Antithrombotic therapy for patients with left ventricular mural thrombus. J Am Coll Cardiol. 2020;75:1676–1685. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez B, James S, Agewall S, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 3.O'Gara PT, Kushner FG, Ascheim DD, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. 2013;127:e362–425. [DOI] [PubMed] [Google Scholar]
- 4.Dalia T, Lahan S, Ranka S, et al. Warfarin versus direct oral anticoagulants for treating left ventricular thrombus: a systematic review and meta-analysis. Thromb J. 2021;19:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilli M, Lombardi M, Del Buono MG, et al. Direct oral anticoagulants vs. vitamin K antagonists for the treatment of left ventricular thrombosis: a systematic review of the literature and meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2021;7:e21–e5. [DOI] [PubMed] [Google Scholar]
- 6.Bass ME, Kiser TH, Page RL, et al. Comparative effectiveness of direct oral anticoagulants and warfarin for the treatment of left ventricular thrombus. J Thromb Thrombolysis. 2021;52:517–522. [DOI] [PubMed] [Google Scholar]
- 7.Iskaros O, Marsh K, Papadopoulos J, et al. Evaluation of direct oral anticoagulants versus warfarin for intracardiac thromboses. J Cardiovasc Pharmacol. 2021;77:621–631. [DOI] [PubMed] [Google Scholar]
- 8.Willeford A, Zhu W, Stevens C, et al. Direct oral anticoagulants versus warfarin in the treatment of left ventricular thrombus. Ann Pharmacother. 2021;55:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z, Li X, Li X, et al. Direct oral anticoagulants versus vitamin K antagonists for patients with left ventricular thrombus. Ann Palliat Med. 2021;10:9427–9434. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Si D, Zhang Q, et al. Rivaroxaban versus Vitamin K Antagonists (warfarin) based on the triple therapy for left ventricular thrombus after ST-Elevation myocardial infarction. Heart Vessels. 2022;37:374–384. [DOI] [PubMed] [Google Scholar]
- 11.Abdelnabi M, Saleh Y, Fareed A, et al. Comparative study of oral anticoagulation in left ventricular thrombi (No-LVT trial). J Am Coll Cardiol. 2021;77:1590–1592. [DOI] [PubMed] [Google Scholar]
- 12.Alcalai R, Butnaru A, Moravsky G, et al. Apixaban versus warfarin in patients with left ventricular thrombus, A prospective multicenter randomized clinical trial. Eur Heart J Cardiovasc Pharmacother. 2021; doi: 10.1093/ehjcvp/pvab057. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Haniff W. ClinicalTrials.gov [database online]. Penang, Malaysia: Universiti Sains; 2016. Updated July 8, 2021.
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 17.Cochran JM, Jia X, Kaczmarek J, et al. Direct oral anticoagulants in the treatment of left ventricular thrombus: a retrospective, multicenter study and meta-analysis of existing data. J Cardiovasc Pharmacol Ther. 2021;26:173–178. [DOI] [PubMed] [Google Scholar]
- 18.Ali Z, Isom N, Dalia T, et al. Direct oral anticoagulant use in left ventricular thrombus. Thromb J. 2020;18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daher J, Da Costa A, Hilaire C, et al. Management of left ventricular thrombi with direct oral anticoagulants: retrospective comparative study with vitamin K antagonists. Clin Drug Investig. 2020;40:343–353. [DOI] [PubMed] [Google Scholar]
- 20.Guddeti RR, Anwar M, Walters RW, et al. Treatment of left ventricular thrombus with direct oral anticoagulants: a retrospective observational study. Am J Med. 2020;133:1488–1491. [DOI] [PubMed] [Google Scholar]
- 21.Iqbal H, Straw S, Craven TP, et al. Direct oral anticoagulants compared to vitamin K antagonist for the management of left ventricular thrombus. ESC Heart Fail. 2020;7:2032–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones DA, Wright P, Alizadeh MA, et al. The use of novel oral anticoagulants compared to vitamin K antagonists (warfarin) in patients with left ventricular thrombus after acute myocardial infarction. Eur Heart J Cardiovasc Pharmacother. 2021;7:398–404. [DOI] [PubMed] [Google Scholar]
- 23.Robinson AA, Trankle CR, Eubanks G, et al. Off-label use of direct oral anticoagulants compared with warfarin for left ventricular thrombi. JAMA Cardiol. 2020;5:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massussi M, Scotti A, Lip GYH, et al. Left ventricular thrombosis: new perspectives on an old problem. Eur Heart J Cardiovasc Pharmacother. 2021;7:158–167. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy CP, Vaduganathan M, McCarthy KJ, et al. Left ventricular thrombus after acute myocardial infarction: screening, prevention, and treatment. JAMA Cardiol. 2018;3:642–649. [DOI] [PubMed] [Google Scholar]
- 26.Cambronero-Cortinas E, Bonanad C, Monmeneu JV, et al. Incidence, outcomes, and predictors of ventricular thrombus after reperfused ST-segment-elevation myocardial infarction by using sequential cardiac MR imaging. Radiology. 2017;284:372–380. [DOI] [PubMed] [Google Scholar]
- 27.Bulluck H, Chan MHH, Paradies V, et al. Incidence and predictors of left ventricular thrombus by cardiovascular magnetic resonance in acute ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: a meta-analysis. J Cardiovasc Magn Reson. 2018;20:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maniwa N, Fujino M, Nakai M, et al. Anticoagulation combined with antiplatelet therapy in patients with left ventricular thrombus after first acute myocardial infarction. Eur Heart J. 2018;39:201–208. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy CP, Murphy S, Venkateswaran RV, et al. Left ventricular thrombus: contemporary etiologies, treatment strategies, and outcomes. J Am Coll Cardiol. 2019;73:2007–2009. [DOI] [PubMed] [Google Scholar]
- 30.Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
- 31.Fanaroff AC, Ohman EM. Non-vitamin K antagonist oral anticoagulants in the treatment of atrial fibrillation. Annu Rev Med. 2019;70:61–75. [DOI] [PubMed] [Google Scholar]
- 32.Gibson CM, Mehran R, Bode C, et al. An open-label, randomized, controlled, multicenter study exploring two treatment strategies of rivaroxaban and a dose-adjusted oral vitamin K antagonist treatment strategy in subjects with atrial fibrillation who undergo percutaneous coronary intervention (PIONEER AF-PCI). Am Heart J. 2015;169:472–478.e5. [DOI] [PubMed] [Google Scholar]
- 33.Peterson BE, Bhatt DL, Gabriel Steg P, et al. Evaluation of dual versus triple therapy by landmark analysis in the RE-DUAL PCI trial. JACC Cardiovasc Interv. 2021;14:768–780. [DOI] [PubMed] [Google Scholar]
- 34.Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. [DOI] [PubMed] [Google Scholar]
- 35.Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335–1343. [DOI] [PubMed] [Google Scholar]
- 36.Caturano A, Galiero R, Pafundi PC. Atrial fibrillation and stroke. A review on the use of vitamin K antagonists and novel oral anticoagulants. Medicina (Kaunas). 2019;55:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.