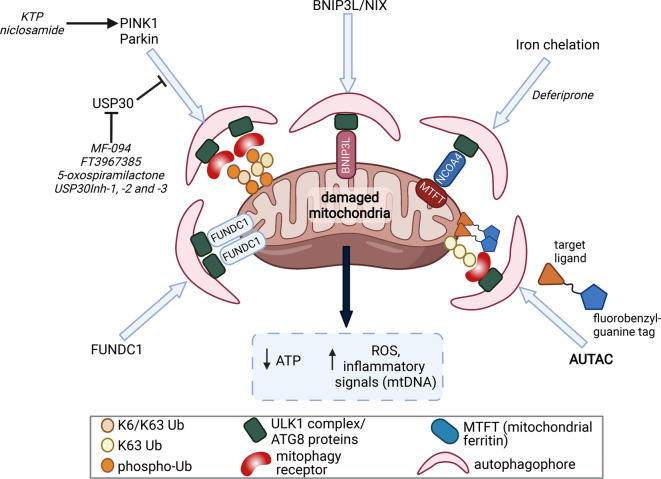
Figure 1. PINK1/Parkin-dependent mitophagy.
Following mitochondrial damage due to protein misfolding or electron transport chain decouplers, PINK1 is stabilised on the outer mitochondrial membrane (OMM) and activates itself through trans-autophosphorylation. PINK1 then phosphorylates ubiquitin conjugated to mitochondrial proteins such as VDAC1 and mitofusin 2. Parkin translocates to sites of phospho-ubiquitin accumulation and is completely activated following its binding to phospho-ubiquitin and phosphorylation by PINK1. Fully active Parkin then catalyses the addition of ubiquitin via its catalytic cysteine (Cys431; indicated by yellow star) onto OMM proteins predominantly through K63 and K6 linkages. Ubiquitin can then be further phosphorylated via PINK1 to recruit more Parkin thus leading to a feed-forward amplification loop to mark the OMM quickly and efficiently with ubiquitin chains. This ubiquitination is opposed by the mitochondrial deubiquitinating enzyme USP30. The nascent ubiquitin chains are recognised by mitophagy receptors such as optineurin and NDP52. These mitophagy receptors recruit the ULK1 complex at focal points on mitochondria to initiate autophagophore formation with the subsequent fusion with lysosomes ultimately leading to autophagic degradation of damaged mitochondria via lysosomal hydrolases.