Abstract
Aim
Exposure to prenatal opioids may adversely impact the developing brain networks. The aim of this pilot study was to evaluate alterations in amygdalar functional connectivity in human infants with prenatal opioid exposure.
Methods
In this prospective IRB approved study, we performed resting state functional MRI (rs-fMRI) in 10 infants with prenatal opioid exposure and 12 infants without prenatal drug exposure at <48 weeks corrected gestational age. Following standard pre-processing, we performed seed based functional connectivity analysis with the right and left amygdala as the regions of interest after correcting for maternal depression and infant sex. We compared functional connectivity of the amygdala network between infants with and without prenatal opioid exposure.
Results
There were significant differences in connectivity of the amygdala seed regions to the several cortical regions including the medial prefrontal cortex in infants who had prenatal opioid exposure when compared to opioid naïve infants.
Conclusion
This finding of increased amygdala functional connectivity in infants with in-utero opioid exposure suggests a potential role of maternal opioid exposure on infants’ altered amygdala function. This association with pre-natal exposure needs to be replicated in future larger studies.
Keywords: Neonatal abstinence syndrome, addiction, neurodevelopment, functional connectivity
Introduction
There has been a dramatic increase in the number of pregnant women using opioids over the past two decades, resulting in corresponding increase in the number of neonates born with prenatal opioid exposure.[1–3] In addition to the early neonatal withdrawal symptoms, prenatal opioid exposure is known to cause long term adverse effects on childhood cognition, development and behavior which are likely a combination of opioid related changes in the fetal and neonatal brain along with other maternal, social and environmental factors. Early neuroimaging could provide information on developmental alterations in brain structure and function in prenatal opioid exposure with potential for neurodevelopmental prognostication. [4–7] Methods to study the extent of impact of opioids on fetal and neonatal brain development and their long-term consequences would enable creation of programs to improve childhood developmental outcomes in these infants.
Early brain developmental alterations have been well described in prenatal exposure to non-opioid drugs, especially methamphetamine, cocaine, alcohol and nicotine.[8–10] Specifically, these groups have shown alterations in amygdala functional connections to the frontal lobe in marijuana, cocaine and polydrug exposure.[11,12] There is only a limited published information on structural changes to developing brain with prenatal opioid exposure [13–15] and there are no functional connectivity study of infants exposed to in-utero opioids. Therefore, in this study, we aimed to evaluate alterations in brain functional connectivity of the amygdala specifically to the frontal lobe in infants with prenatal opioid exposure compared to healthy infants without prenatal drug exposure. Because of existing literature in infants with prenatal opioid and polysubstance exposure showing functional alterations in the amygdala to prefrontal connectivity [12], we specifically investigated for this relationship in our cohort as well.
Methods
Indiana University School of Medicine Institutional Review Board approved this prospective ongoing study of mothers using opioids during pregnancy and their infants. Written informed consent was obtained from the parent for all minor participants. Ten term born infants with prenatal opioid exposure and 13 healthy infants without any prenatal drug exposure were studied at less than 48 weeks corrected gestational age. Infants who had any findings of genetic or major congenital anomalies, or significant postnatal abnormalities such as birth asphyxia or neonatal sepsis were not enrolled. Medical records and maternal self-report questionnaire were used to collect information regarding maternal opioid use, maternal depression, infant birth and postnatal details, and demographics.
MR Data acquisition
All MR imaging was performed on the same research-dedicated 3 Tesla Siemens Prisma (Erlangen, Germany) on infants during natural sleep with feed and swaddle technique and using a 64-channel head coil [16]. Sleeping infants were swaddled using Med-Vac device (Domico Med device). Mothers of infants were welcome to stay with the infant in the MR scan room if they wished. Appropriate ear protection with earplugs and earmuffs (Natus minimuffs) was provided for the infants. Standard ear protection was provided for mothers if they were in the scan room. Structural imaging included high resolution 3D T1-weighted MPGRAGE sequence at 1 × 1 × 1 mm resolution (TR= 2010 ms, TE= 2.91 ms, TI=1610 ms, flip angle= 12°, 192 mm field-of-view, 120 slices, and GRAPPA acceleration iPAT= 2) and high-resolution axial T2-weighted imaging with a Turbo-Spin-Echo pulse sequence at high in-plane resolution (0.9 × 0.9 mm, 1 mm slice thickness, TR=9550ms, TE= 145ms). Blood oxygen level dependent (BOLD) contrast resting-state functional MRI (rfMRI) was performed using a single-shot EPI sequence with a multiband factor of 3, TR=1205 ms, TE=30.4 ms, 480 volumes with an isotropic resolution of 2.5mm, and 9:45 min acquisition time. Total scan time was 22 minutes although actual time spent in scanner was typically longer for positioning or calming the infant.
MR processing
Preprocessing of the MR images for each subject was performed using the FMRIB (for Functional MRI of the Brain) software Library (FSL, Oxford, UK) [17]. Skull stripping and removal of non-brain tissue in T2 weighted anatomic images and BOLD contrast images were performed using brain tissue segmentation using FSL tool (BET) [18]. The first 10 volumes from the rs-fMRI BOLD sequence were excluded due to non-steady state of the MR signal [19]. Geometric distortions due to B0 field inhomogeneity were corrected by using the FSL-topup. [20] Global intensity normalization was performed across the time series. The head motion parameters (volume-to-reference transform matrices, and corresponding rotation and translation parameters) of the rs-fMRI datasets were estimated, and realigned using linear motion correction through FSL-MCFLIRT [21]. Spatial smoothing (Gaussian kernel FWHM of 5 mm), and regression of white matter, CSF signal and 6 motion parameters were obtained. In addition, second order motion derivatives were obtained and thresholded with a framewise displacement threshold of 0.3 and DVARS of 3 [22]. Band-pass temporal filtering was applied to remove low frequency scanner drift and physiologic and vascular oscillations. Regions of interest corresponding to the right and left amygdala were extracted from the UNC neonate atlas template space [23] and warped to the subject space using ANTs [24].For each seed region, mean time series was calculated by averaging across all the voxels within the region. BOLD data for each subject was then registered to the subject space T2W images and then to the UNC neonatal template space [24]. The UNC neonatal template is a 3D group template of T2 weighted brain images of 95 neonates [23]. GLM seed based whole brain functional connectivity analysis was performed for average BOLD time series in the right and left amygdala separately using the motion parameters and confound matrix of the FD and DVARS as nuisance variables [25,26]. This produced individual subject level maps of brain regions correlating with the amygdala seed ROIs. Independent mixed-effects group analyses [27] between opioid exposed and controls were then conducted for each amygdala seed region using infant sex and maternal depression as covariates as both these factors are shown to impact amygdala connectivity [28]. We did not include gestational age and age at MRI as covariates as they were not significantly different between the groups. Cluster based corrections for multiple comparisons was performed using an FDR corrected cluster p value of <0.05.
Since prior studies in infants with prenatal cocaine exposure have suggested a drug common area of altered amygdala connectivity in the medial prefrontal region [12], we also investigated any medial prefrontal alterations in amygdala connectivity for correlation with opioid dose for infants of mothers that were on medication assisted treatment with buprenorphine or methadone using the morphine milligram equivalent dose (https://www.cms.gov/Medicare/Prescription-Drug-coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf).
Results
Demographics:
We recruited 29 infants, of whom 22 infants were included in the final analysis (Figure 1). There were 10 prenatal opioid exposed infants and 12 opioid naïve infants. Demographic information is provided in Table 1. Both groups were comparable with no significant difference in gestational age at birth, age at MRI, sex proportion and the APGAR scores at 1 and 5 minutes. The opioid exposed infants had a significantly lower birth weight and longer length of hospital stay compared to the non-opioid exposed infants. Nine of the 10 opioid using mothers were undergoing medication assisted treatment; seven were on buprenorphine therapy and two were on methadone replacement therapy. There were four opioid exposed mothers who used other substances during pregnancy, including marijuana, amphetamines, cocaine, cannabis and heroin. Seven of the 10 mothers also smoked during pregnancy, while none of the control mothers smoked during pregnancy.
Figure 1:
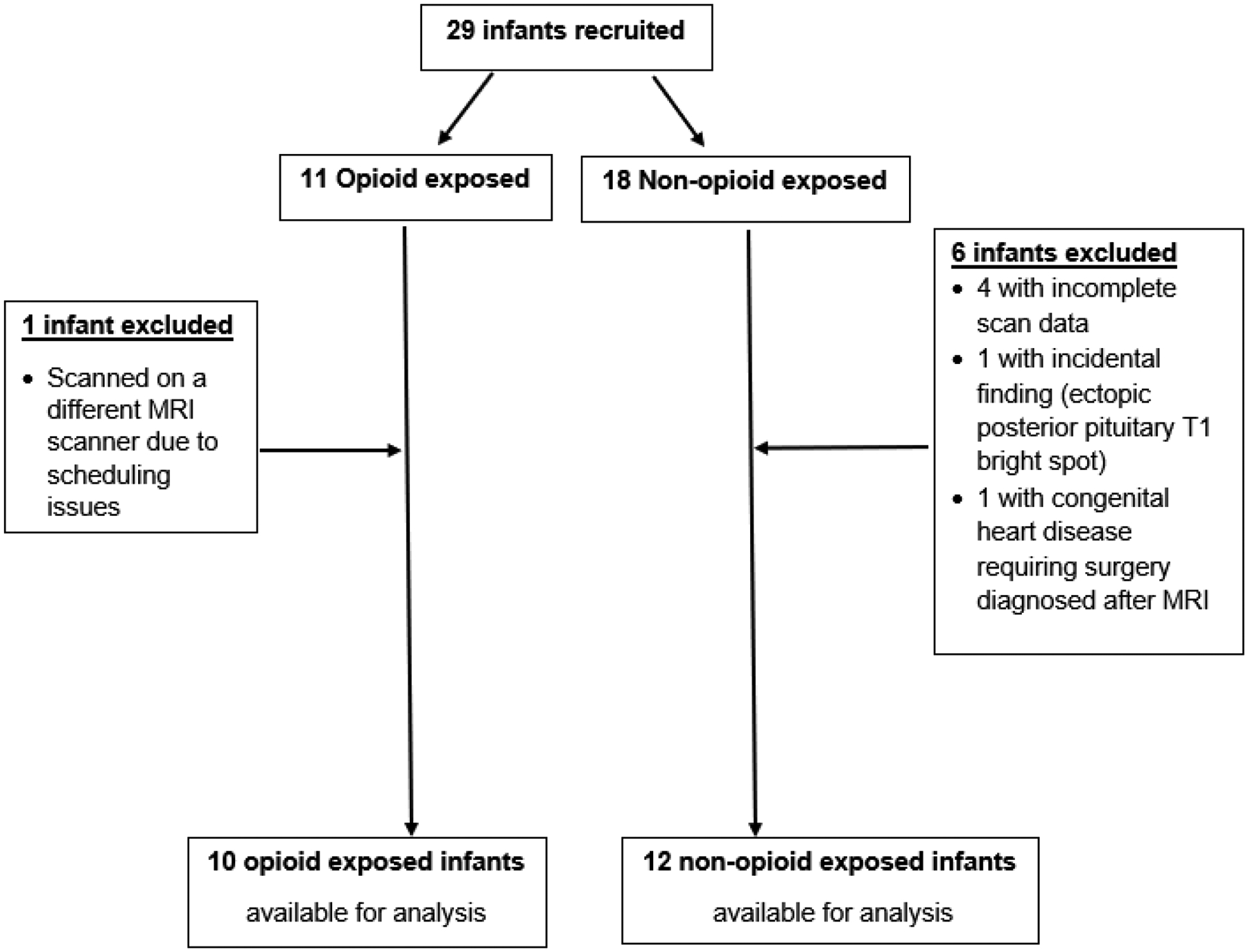
Flowchart of subject selection
Table 1.
Demographics
| Prenatal Opioid Exposed infants N = 10 | Non-opioid Exposed infants N = 12 | P-value (t test) | |
|---|---|---|---|
| Gestational age in weeks, Mean (SD) | 38.48 (1.17) | 39.37 (0.81) | 0.07 |
| Age at MRI in days, Mean (SD) | 31.9 (14) | 28.5 (14.83) | 0.61 |
| Sex | 8 Female: 2 Male | 6 Female: 6 Male | 0.20† |
| Maternal depression (N) | 4 | 1 | 0.14† |
| Need for postnatal opioid treatment (N) | 3 | N/A | N/A |
| APGAR 1 min, Mean | 8.56 | 8.25 | 0.51 |
| APGAR 5 min, Mean | 8.78 | 8.83 | 0.84 |
| Birth weight in Kg, Mean (SD) | 2.84 (0.25) | 3.34 (0.27) | < 0.001 |
| Length of hospital stay, Mean (SD) | 7.7 (4.73) | 2 (0.91) | <0.01 |
Fisher exact Chi Square test
Amygdala seed-based connectivity:
Figures 2 shows the regions of significantly correlated connectivity associated with the right and left amygdala seed in the frontal, temporal, parietal, occipital regions and cerebellum. We identified overlapping regions of increased connectivity in the medial prefrontal region, for the right and left amygdala seed maps, with significantly greater connectivity in the opioid exposed infants compared to the control infants (Figure 3). Significant regions of cluster Z score values for the right and left amygdala are listed in Table 2. There were no significant clusters that were higher in the control compared to the prenatal opioid exposed group for the left amygdala.
Figure 2:
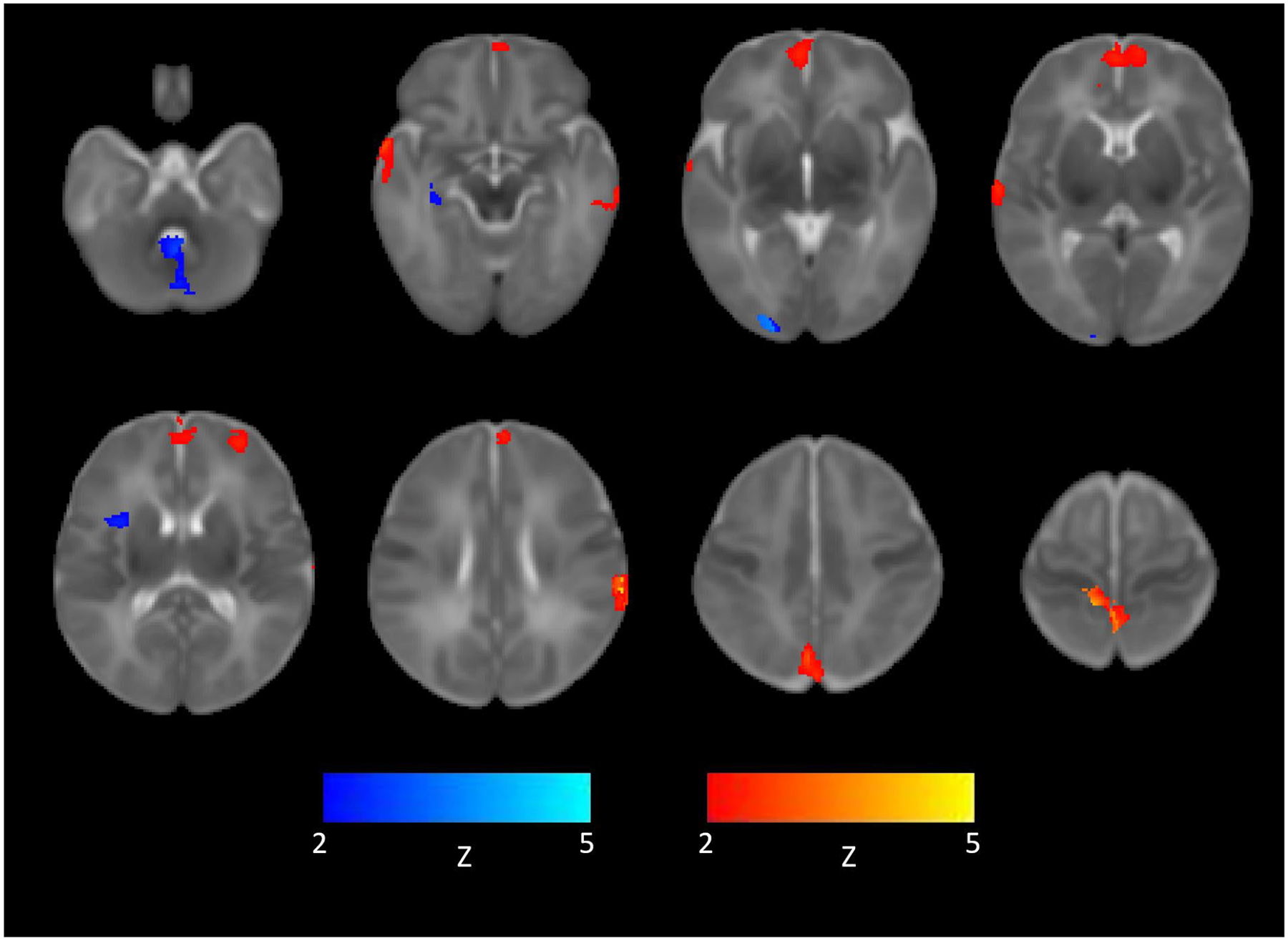
Representative images of how right and left amygdala seed region connectivity differed between the opioid exposed and opioid non-exposed groups. Warm colors indicate greater signal in opioid exposed compared to opioid non-exposed infants. Cooler colors indicate greater signal in opioid non-exposed compared to opioid exposed infants. Displayed in radiologic convention at FDR corrected cluster p ≤ 0.05.
Figure 3a:
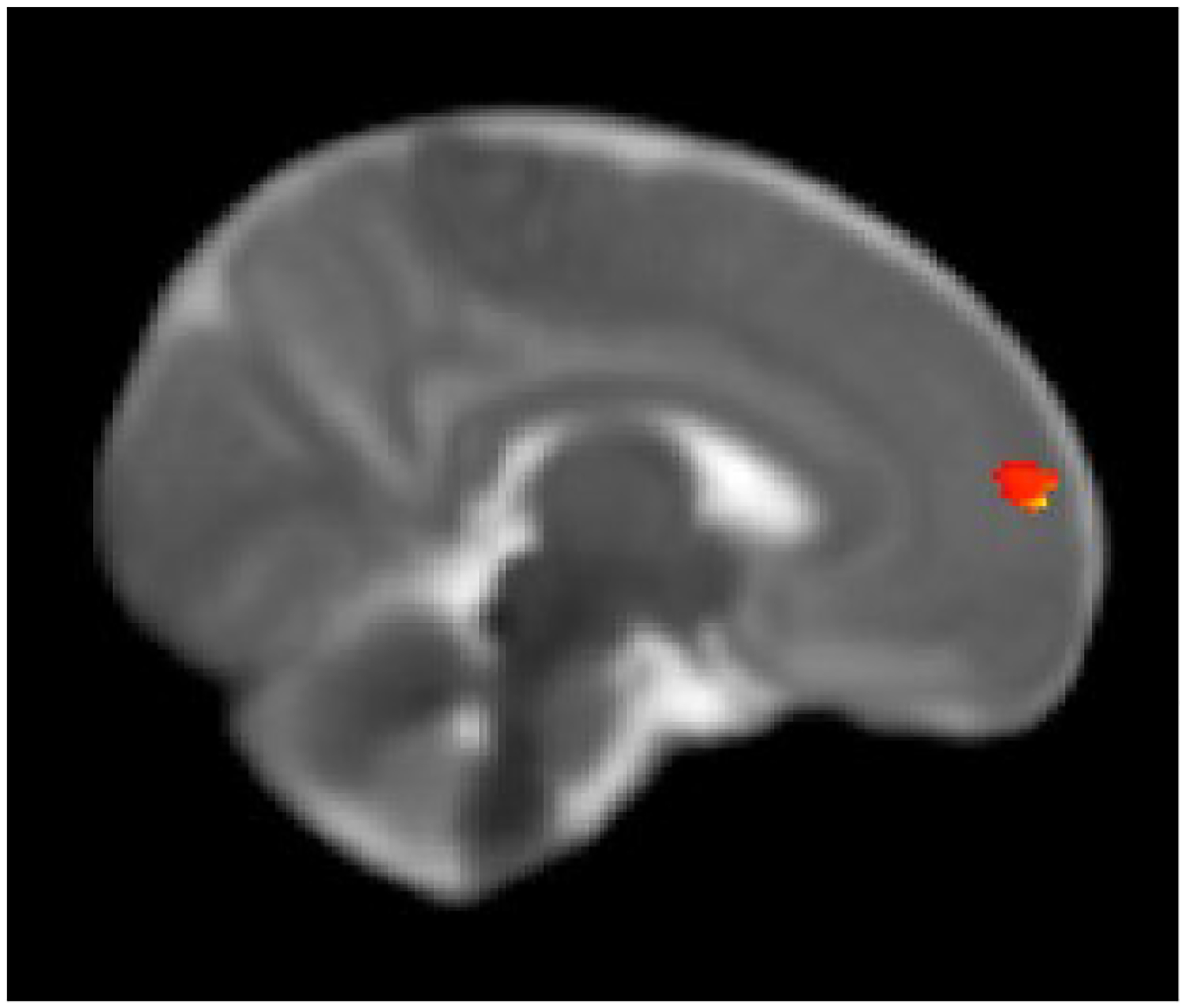
Right frontal cluster showing higher functional connectivity with the seed region in the right amygdala in infants with prenatal opioid exposure compared to non-opioid exposed infants. Displayed on the University of North Carolina (UNC) neonatal template at FDR corrected cluster p ≤ 0.05.
Table 2.
FDR cluster corrected Z-score values of significant regions
| Region | Coordinates of max Z-score | Max Z score | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Prenatal opioid exposure higher than control for L amygdala | ||||
| R medial prefrontal | 3 | 36 | 7 | 3.42 |
| L Inf temporal gyrus | −31 | −33 | −16 | 2.57 |
| R anterior temporal | 33 | 2 | 97 | 4.07 |
| Bil paracentral lobule | 9 | −30 | 41 | 4.35 |
| L Inf parietal lobule | −44 | −28 | 19 | 4.74 |
| Bil precuneus | 3 | −20 | 1 | 3.35 |
| R Sup temporal gyrus | 44 | −13 | 1 | 2.48 |
| Prenatal opioid exposure higher than control for R amygdala | ||||
| Bil medial prefrontal | −6 | 33 | 9 | 2.44 |
| L dorsal prefrontal | −20 | 28 | 13 | 2.38 |
| L inf parietal lobule | −44 | −27 | 20 | 2.48 |
| R precuneus | 4 | −54 | 32 | 2.71 |
| L middle temporal gyrus | −33 | −22 | −5 | 2.35 |
| Control higher than prenatal opioid exposure for L amygdala | ||||
| R anterior insula | 24 | 0 | 12 | 2.4 |
| R medial temporal | 23 | −19 | −4 | 3.02 |
| R occipital | 14 | −64 | 1 | 3.87 |
| Cerebellar vermis | 0 | −31 | 20 | 2.85 |
There were no significant correlations between morphine milligram equivalent dose with frontal areas of significant amygdalar connectivity (Figure 3) in infants of mothers on medication assisted treatment.
Discussion
We report new findings of altered connectivity of the amygdala to several cortical regions of the brain in infants within prenatal opioid exposure compared to nondrug exposed infants. The amygdala is important in regulation of emotion, fear, stress response, motivation and behavior. Early insult to the developing infant amygdala and its functional connections have been shown to be associated with poor behavioral outcomes [29–31]. So far, existing studies in prenatal opioid exposure related brain functional connectivity are mainly in adolescents and the strength of our study is in assessing the infant brain early in life, before the influence of environmental effects [32].
Of the different regions of amygdala to cortical altered connectivity, there were overlapping regions of higher medial prefrontal connectivity in POE compared to the nondrug exposed infants. This finding is concordant with previous investigations in infants with prenatal polysubstance and cocaine exposure in which higher amygdala to medial prefrontal cortex connection was higher in drug exposed infants compared to healthy controls [12]. Impairment in normal inhibition of the amygdala by the frontal lobe due to drug exposure has been proposed as a mechanism for this increased connectivity of the amygdala to the frontal lobes [12]. The medial prefrontal cortex is an important component of the executive function and working memory, and therefore alterations in this regional connectivity may have the potential to influence long term outcomes in prenatal opioid exposure [33,32]. In older children with prenatal opioid exposure, impaired performance on working memory and selective attention tasks was shown and was associated increased activation of the prefrontal cortical regions during performance of the working memory task compared to non-drug exposed controls [34]. In our study, we did not find any correlation between medial prefrontal connectivity with dose of opioid used possibly explained by our small sample size and confounding effects of smoking and other drug exposure.
Both the right and left amygdala also showed overlapping regions of higher connectivity to the precuneus in infants with POE when compared to controls. We found significant higher connectivity in the control group to regions of the cortex including the right medial temporal region, right occipital, and frontal and parietal regions, but there were no regions of significant higher connectivity for the right amygdala. Other studies have also shown differences in right and left amygdala connections, and the reason for this lack of symmetry, while not exactly known, may be hypothesized to be due to varying developmental trajectories of the amygdala. Other researchers have also shown regions of cortical connectivity to the amygdala that overlap with our findings. Connectivity of the amygdala to the precuneus and the supramarginal gyrus (part of the inferior parietal lobule) have been shown to be associated with maternal cortisol and infant sex interaction, with the sex specific amygdala to supramarginal gyrus connectivity found to be associated with internalizing behaviors at 24 months [29]. Similarly, infants of pregnant women with higher systemic interlukin-6, a marker of maternal inflammation, were associated with larger right amygdala volume and stronger bilateral amygdala connectivity to the fusiform gyrus, somatosensory cortex and thalamus, anterior insula and caudate and parahippocampal gyrus [30]. The larger right amygdala volume and stronger left amygdala connectivity in these infants were also found to be been associated with lower impulse control at 24 months and mediated the association between higher maternal interlukin-6 concentrations and lower impulse control [30]. In infants born to mothers with maternal depression, graph theory analysis of resting state functional MRI showed higher provincial hub values in the amygdala compared to healthy controls; these hub values predicted temperament at 6 months.[31]
Although our study reports novel associations of brain connectivity with prenatal opioid exposure, there are a few limitations and the following caveats should be considered when interpreting the findings. First, this is a pilot study with a small sample size; a larger study is needed to replicate the findings and extend analysis to other brain regions. Second, although we accounted for known confounders such as maternal depression and infant sex as covariates in our analysis and demonstrated persistent alterations in amygdala connectivity, a larger study is needed to account for all potential confounders and environmental variables such as maternal smoking, other drug use, nutritional status, stress and socioeconomic status, that could also synergistically affect the brain functional connectivity. Third, the study’s findings of altered amygdala to cortical connectivity in the context of prenatal opioid exposure need to be correlated with clinical neurodevelopment and behaviors to better understand the long-term clinical implications. Given the presence of neuroplasticity of the developing brain, it is important to understand whether these early changes in brain function and any putative associated clinical outcomes would persist in childhood or adolescence, and to what extent they may be modulated by early and targeted therapies. All these areas would be interesting areas for future research. Additional avenues of inquiry should attempt to identify specific genetic factors that may modulate the effect of prenatal opioid exposure and should include identification of targeted early interventions to mitigate the impact of prenatal opioid exposure on the developing brain.
Conclusion
There are alterations in amygdala to cortical connectivity in infants with prenatal opioid exposure compared to non-opioid exposed infants. Future large clinical studies are needed to validate our new findings, further study the impact of prenatal maternal opioid exposure on amygdala connectivity and other regions of brain in infants and young children and understand the long-term clinical neurodevelopmental and behavioral implications of increased amygdala-frontal cortex connectivity with prenatal opioid exposure.
Figure 3b:
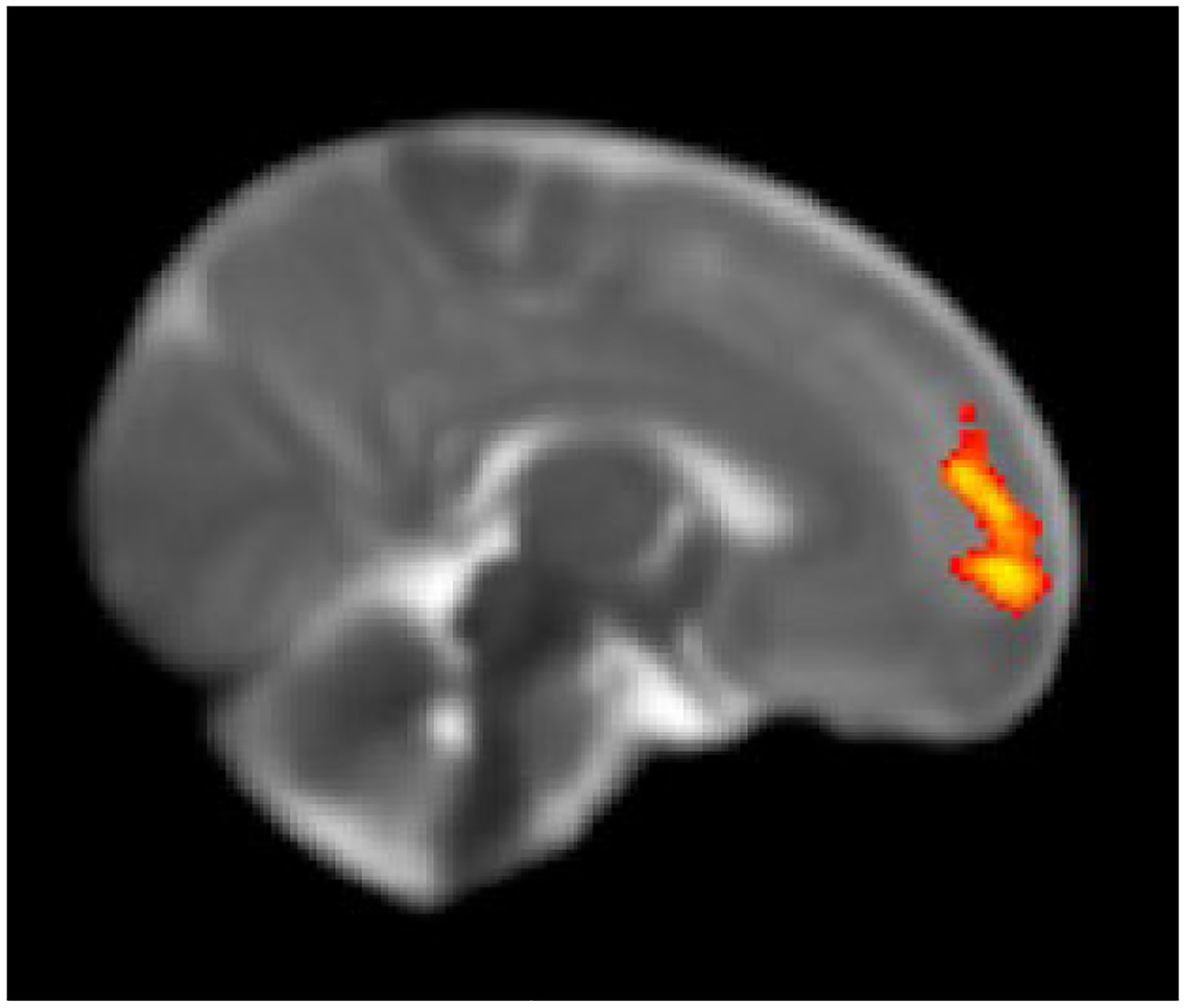
Right frontal cluster showing increased functional connectivity of the seed region with the left amygdala in infants with prenatal opioid exposure compared to non-opioid exposed infants. Displayed on the UNC neonatal template FDR corrected cluster p ≤ 0.05.
Funding Sources:
RR was supported by the American Roentgen Ray Scholarship Award 2018 and Radiological Society of North America Seed Grant 2018. SS and RR were supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award, R01HD096800 (PI: Sadhasivam). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. For minor participants, informed consent was obtained from the guardian.
References
- 1.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF (2014) Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol 123 (5):997–1002. doi: 10.1097/AOG.0000000000000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick SW, Davis MM, Lehmann CU, Cooper WO (2015) Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol 35 (8):650–655. doi: 10.1038/jp.2015.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM (2012) Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA 307 (18):1934–1940. doi: 10.1001/jama.2012.3951 [DOI] [PubMed] [Google Scholar]
- 4.Ross EJ, Graham DL, Money KM, Stanwood GD (2015) Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology 40 (1):61–87. doi: 10.1038/npp.2014.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romanowicz M, Vande Voort JL, Shekunov J, Oesterle TS, Thusius NJ, Rummans TA, Croarkin PE, Karpyak VM, Lynch BA, Schak KM (2019) The effects of parental opioid use on the parent-child relationship and children’s developmental and behavioral outcomes: a systematic review of published reports. Child Adol Psych Men 13. doi:ARTN 5 10.1186/s13034-019-0266-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nygaard E, Slinning K, Moe V, Due-Tonnessen P, Fjell A, Walhovd KB (2018) Neuroanatomical characteristics of youths with prenatal opioid and poly-drug exposure. Neurotoxicol Teratol 68:13–26. doi: 10.1016/j.ntt.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 7.Mayes LC, Granger RH, Bornstein MH, Zuckerman B (1992) The problem of prenatal cocaine exposure. A rush to judgment. JAMA 267 (3):406–408 [PubMed] [Google Scholar]
- 8.Upton MN, Smith GD, McConnachie A, Hart CL, Watt GC (2004) Maternal and personal cigarette smoking synergize to increase airflow limitation in adults. Am J Respir Crit Care Med 169 (4):479–487. doi: 10.1164/rccm.200211-1357OC [DOI] [PubMed] [Google Scholar]
- 9.Stroud LR, Paster RL, Goodwin MS, Shenassa E, Buka S, Niaura R, Rosenblith JF, Lipsitt LP (2009) Maternal smoking during pregnancy and neonatal behavior: a large-scale community study. Pediatrics 123 (5):e842–848. doi: 10.1542/peds.2008-2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, Beaulieu C (2013) Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J Neurosci 33 (24):10098–10109. doi: 10.1523/JNEUROSCI.5004-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grewen K, Salzwedel AP, Gao W (2015) Functional Connectivity Disruption in Neonates with Prenatal Marijuana Exposure. Front Hum Neurosci 9:601. doi: 10.3389/fnhum.2015.00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salzwedel AP, Grewen KM, Vachet C, Gerig G, Lin W, Gao W (2015) Prenatal drug exposure affects neonatal brain functional connectivity. J Neurosci 35 (14):5860–5869. doi: 10.1523/JNEUROSCI.4333-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walhovd KB, Watts R, Amlien I, Woodward LJ (2012) Neural tract development of infants born to methadone-maintained mothers. Pediatr Neurol 47 (1):1–6. doi: 10.1016/j.pediatrneurol.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 14.Yuan Q, Rubic M, Seah J, Rae C, Wright IM, Kaltenbach K, Feller JM, Abdel-Latif ME, Chu C, Oei JL, group BOBC (2014) Do maternal opioids reduce neonatal regional brain volumes? A pilot study. J Perinatol 34 (12):909–913. doi: 10.1038/jp.2014.111 [DOI] [PubMed] [Google Scholar]
- 15.Bier JB, Finger AS, Bier BA, Johnson TA, Coyle MG (2015) Growth and developmental outcome of infants with in-utero exposure to methadone vs buprenorphine. J Perinatol 35 (8):656–659. doi: 10.1038/jp.2015.22 [DOI] [PubMed] [Google Scholar]
- 16.Antonov NK, Ruzal-Shapiro CB, Morel KD, Millar WS, Kashyap S, Lauren CT, Garzon MC (2017) Feed and Wrap MRI Technique in Infants. Clin Pediatr (Phila) 56 (12):1095–1103. doi: 10.1177/0009922816677806 [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) Fsl. Neuroimage 62 (2):782–790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 18.Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17 (3):143–155. doi: 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao-Gan Y, Yu-Feng Z (2010) DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front Syst Neurosci 4:13. doi: 10.3389/fnsys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson J, Skare S (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 20:870–888 [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage 17:825–841 [DOI] [PubMed] [Google Scholar]
- 22.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59 (3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi F, Yap P-T, Wu G, Jia H, Gilmore JH, Lin W, Shen D (2011) Infant Brain Atlases from Neonates to 1- and 2-year-olds. PLoS ONE 6 (4):e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avants B, Tustison N, Song G, Cook P, Klein A, Gee J (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54:2033–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34 (4):537–541. doi: 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- 26.Simons LE, Pielech M, Erpelding N, Linnman C, Moulton E, Sava S, Lebel A, Serrano P, Sethna N, Berde C, Becerra L, Borsook D (2014) The responsive amygdala: treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. Pain 155 (9):1727–1742. doi: 10.1016/j.pain.2014.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mumford JA, Poldrack RA (2007) Modeling group fMRI data. Soc Cogn Affect Neurosci 2 (3):251–257. doi: 10.1093/scan/nsm019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaki M, Nga L, Mather M (2013) Amygdala functional connectivity with medial prefrontal cortex at rest predicts the positivity effect in older adults’ memory. J Cogn Neurosci 25 (8):1206–1224. doi: 10.1162/jocn_a_00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham AM, Rasmussen JM, Entringer S, Ben Ward E, Rudolph MD, Gilmore JH, Styner M, Wadhwa PD, Fair DA, Buss C (2019) Maternal Cortisol Concentrations During Pregnancy and Sex-Specific Associations With Neonatal Amygdala Connectivity and Emerging Internalizing Behaviors. Biol Psychiatry 85 (2):172–181. doi: 10.1016/j.biopsych.2018.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, Potkin SG, Entringer S, Wadhwa PD, Fair DA, Buss C (2018) Maternal Systemic Interleukin-6 During Pregnancy Is Associated With Newborn Amygdala Phenotypes and Subsequent Behavior at 2 Years of Age. Biol Psychiatry 83 (2):109–119. doi: 10.1016/j.biopsych.2017.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotem-Kohavi N, Williams LJ, Muller AM, Abdi H, Virji-Babul N, Bjornson BH, Brain U, Werker JF, Grunau RE, Miller SP, Oberlander TF (2019) Hub distribution of the brain functional networks of newborns prenatally exposed to maternal depression and SSRI antidepressants. Depress Anxiety. doi: 10.1002/da.22906 [DOI] [PubMed] [Google Scholar]
- 32.Schweitzer JB, Riggins T, Liang X, Gallen C, Kurup PK, Ross TJ, Black MM, Nair P, Salmeron BJ (2015) Prenatal drug exposure to illicit drugs alters working memory-related brain activity and underlying network properties in adolescence. Neurotoxicol Teratol 48:69–77. doi: 10.1016/j.ntt.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konijnenberg C, Melinder A (2015) Executive function in preschool children prenatally exposed to methadone or buprenorphine. Child Neuropsychol 21 (5):570–585. doi: 10.1080/09297049.2014.967201 [DOI] [PubMed] [Google Scholar]
- 34.Sirnes E, Griffiths ST, Aukland SM, Eide GE, Elgen IB, Gundersen H (2018) Functional MRI in prenatally opioid-exposed children during a working memory-selective attention task. Neurotoxicol Teratol 66:46–54. doi: 10.1016/j.ntt.2018.01.010 [DOI] [PubMed] [Google Scholar]


