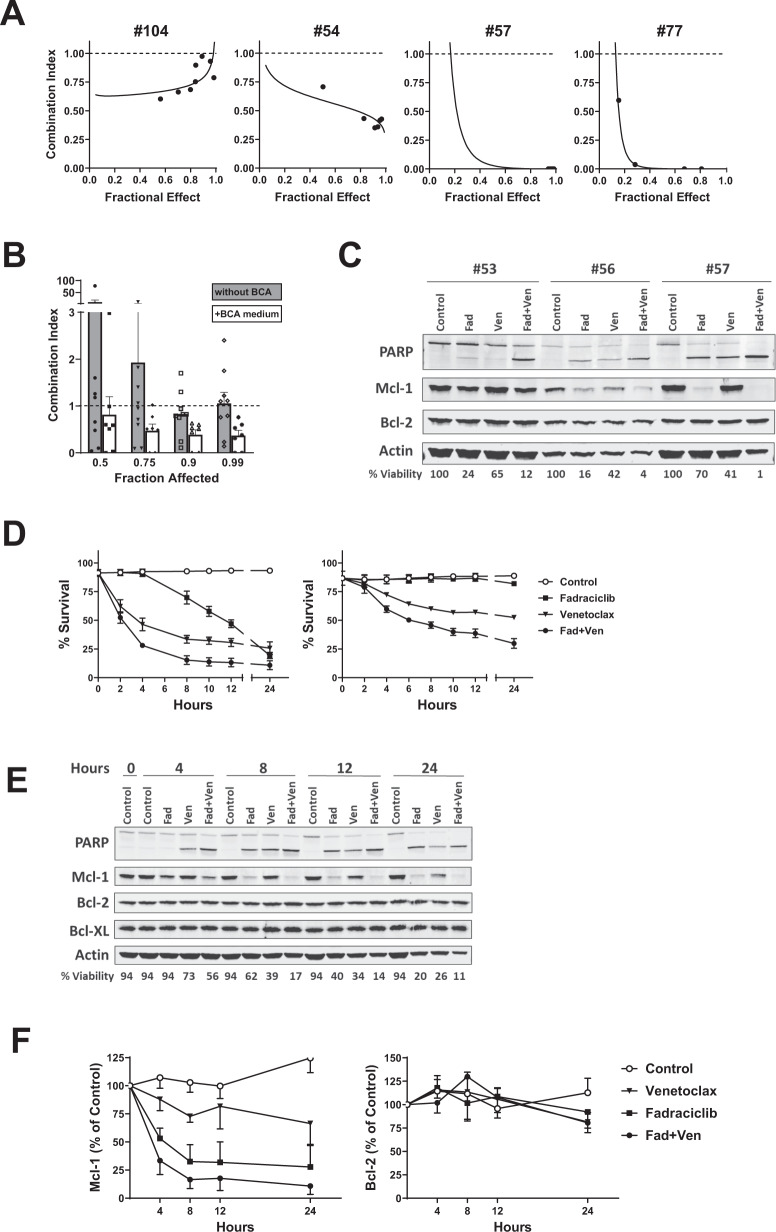
Fig. 5. Synergistic combinations of fadraciclib and venetoclax.
A Representative median-effect plots of the combination of fadraciclib and venetoclax in representative CLL samples cultured without BCA medium (#104) and with BCA medium (#54) and 2 samples with a high percentage of 17p deletion (#57, #77) cultured in BCA medium. B Comparison of the combination index for the combination of fadraciclib and venetoclax in CLL cells cultured without (gray column, 9 samples) or with BCA medium (white column, 7 samples). Data are presented as mean ± SE. C Representative immunoblotting of the combination of fadraciclib (Fad) and venetoclax (Ven) showing PARP cleavage and Mcl-1 and Bcl-2 protein levels. The CLL cells were incubated with 1 X IC50 concentrations of each drug (measured previously for each sample) for 24 h. The percentage of cell viability relative to controls is shown below the images. D Time course of loss of CLL viability induced by fadraciclib, venetoclax, and the combination. CLL cells were incubated with the average IC50 concentrations of fadraciclib (0.8 μM), venetoclax (0.07 μM), and the combination. Cell viability was measured at indicated times up to 24 h. Left: representative of 3 CLL samples without 17p deletion. Right: representative 2 CLL samples with 17p deletion (67% and 95% del17p). E Immunoblot showing the time effect of fadraciclib, venetoclax, and the combination in the samples described in D. F The levels of Mcl-1 (left) and Bcl-2 (right) were quantified in the blots and plotted as percentage of 0-h controls (mean ± SE of 3 samples).