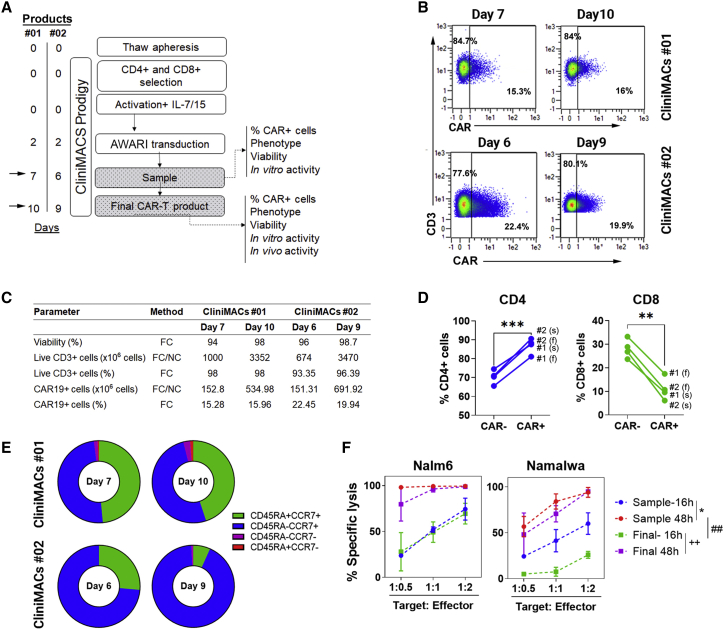
Figure 5.
GMP-like large-scale production of AWARI CAR T cells
(A) Timeline of the GMP-like batches no. 01 and no. 02 of AWARI CAR-T cells in the CliniMACS Prodigy. (B) Dot plots of CAR expression at days of sample (7 and 6) and final product (10 and 9) for both no. 01 (left) and no. 02 (right) batches are shown. (C) Data from viability, number of CD3, efficiency of CAR-T cell transduction, and number of obtained CAR-T cells are shown. FC, flow cytometry; NC, Neubauer chamber counting. (D) Variation of percentage of CD4 and CD8+ cells in CAR− and CAR+ cells at sample (s) and final products (f) is shown. Two-tailed t test is shown. ∗∗p < 0.001; ∗∗∗p < 0.001. (E) Graphical representation of the T cells populations at sample (days 6 or 7) and final products (day 9 or 10) is shown. TN/TSCM, naive and stem cell memory (CD45RA+CCR7+); TCM, central memory (CD45RA−CCR7+); TEM, effector memory (CD45RA−CCR7−); and TEMRA, effector memory RA+ (CD45RA+CCR7−). (F) In vitro specific lysis of CD19+ cells Nalm6 (left) and Namalwa (right) CD19+ cells by AWARI CAR-T cells harvested at sample day (red and blue lines) and final day (green and violet lines) for productions no. 01 and no. 02 is shown. CAR-T cells products were co-cultured at different ratios during 16 h (blue or green lines) or 48 h (red or violet lines). Two-way ANOVA-Tukey’s multiple comparisons are shown.∗p < 0.05; ++p < 0.01; ##p < 0.01.