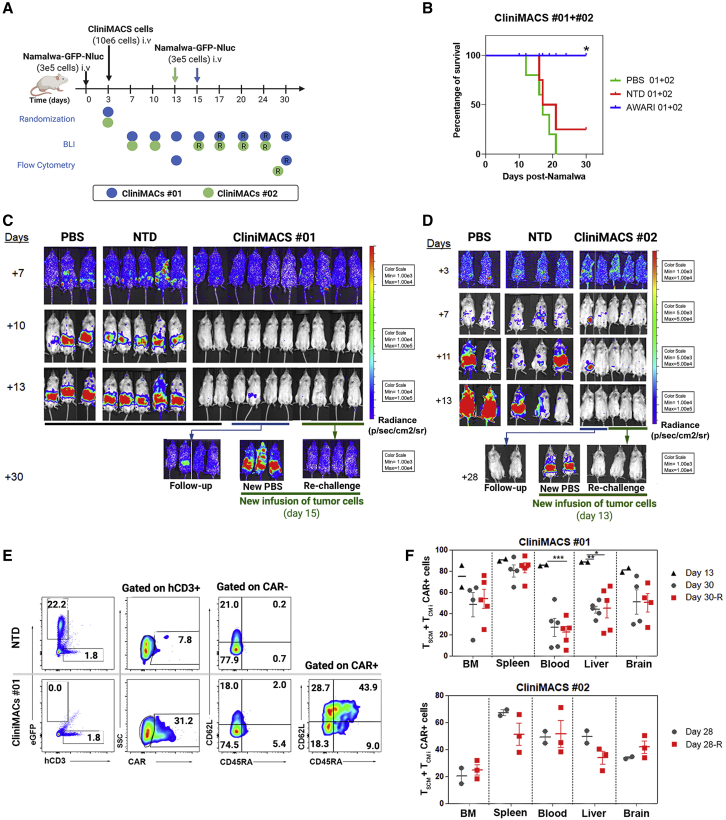
Figure 6.
In vivo anti-tumor activity and phenotype of CliniMACS-produced AWARI CAR-T cells after one or two Namalwa challenges
(A) Experimental design and timeline of the two experiments with CliniMACS batches no. 01 and no. 02. R indicates rechallenge. (B) Survival of PBS, NT, or AWARI-inoculated mice of no. 01 and no. 02 products is shown. Log rank Mantel-Cox test is shown. ∗p < 0.05 (C and D) Representative BLI images of tumor burden measured in no. 01 (C) and no. 02 (D) experiments at different days post-Namalwa inoculation as indicated are shown. In both studies, at day 15 (C) or 13 (D), new Namalwa cells were inoculated to AWARI-treated mice (rechallenge, green line) and to new control mice (“new PBS”). The rest of the treated mice (four AWARI mice in C and two AWARI mice in D) were kept for a follow-up (blue line, follow-up group). (E) Representative dot plots of Namalwa/hCD3 presence, CAR+ cells of hCD3, and phenotype of CAR− and CAR+ of NT cells and AWARI CliniMACS no. 01 from BM after sacrifice are shown. (F) Percentage of TN/SCM + TCM population in hCD3+CAR+ cells analyzed in different organs from mice with one or two (rechallenge, R) inoculations of Namalwa tumor cells in no. 01 (left) and no. 02 (right) CliniMACS batches on the final day is shown. Only when CAR+ population was >1%, data were analyzed and included here. N (CliniMACS no. 01): PBS = 3, NT = 5, and AWARI = 10 (two sacrificed on day 13; four mice with one- and four mice with two challenges). N (CliniMACS no. 02): PBS = 2, NT = 3, and AWARI = 5 (2 + 3 rechallenged mice). Two-way ANOVA and Bonferroni post-test are shown; ∗p < 0.05, ∗∗p < 0.001, and ∗∗∗p < 0.0001.