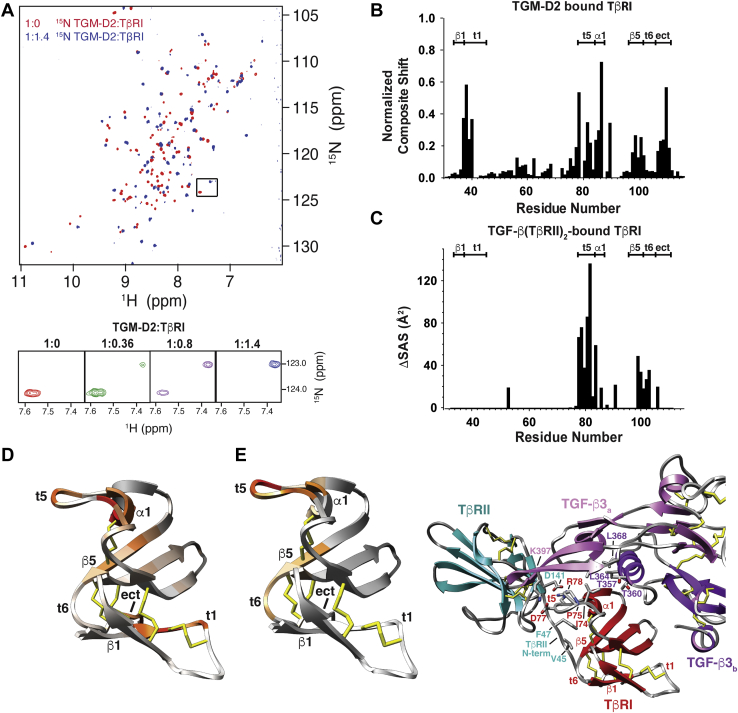
Figure 3.
Binding of TGM-D2 to TβRI.A, 1H-15N HSQC spectra of 0.2 mM 15N TGM-D2 alone (red) overlaid with the spectrum of the same sample, but with 1.2 M equivalents of unlabeled TβRI added (blue). Spectra were recorded in 25 mM sodium phosphate, 50 mM sodium chloride, and 5% 2H2O, pH 7.0, at 310 K. Expansion of the boxed region with intermediate titration points is shown below. B and D, plot of the composite shift perturbations of TβRI upon binding to TGM-D2 (B) and a depiction of these on the structure of TβRI from PDB 2PJY (D). Structure is colored using a scale where white indicates minimal composite shift perturbation and red indicates maximal. C and E, plot of the difference in solvent-accessible surface area for individual residues of TβRI between the free and bound form (PDB 2PJY) (C) and a depiction of these on the structure of TβRI from PDB 2PJY (E, left). Structure is colored using a scale where gray indicates minimal SAS and red indicates maximal SAS. Shown also in (E) (right) is the structure of one side of the TGF-β3(TβRII)2(TβRI)2 complex, with the two monomers of TGF-β3 depicted in pink and magenta, TβRII in cyan, and TβRI in red. Key residues at the interface between TGF-β3:TβRII and TβRI are shown. HSQC, 1H-15N shift correlation.