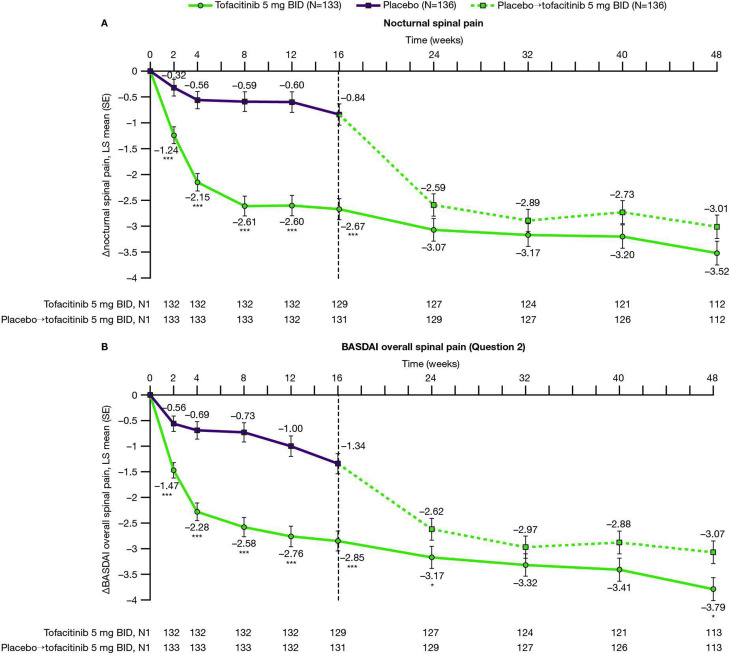
Figure 1.
Changes from baseline to week 48 in (A) nocturnal and (B) BASDAI overall spinal pain. LS mean changes from baseline are shown to week 48 for (A) nocturnal spinal pain and (B) BASDAI overall spinal pain, in patients with AS receiving tofacitinib 5 mg twice daily or placebo→tofacitinib 5 mg twice daily.† Results up to week 16, based on MMRM, include all postbaseline data to week 16 (data cut-off 19 December 2019; data snapshot 29 January 2020); results after week 16 are based on another MMRM including all postbaseline data to week 48 (reporting results after week 16 only). *p≤0.05, **p<0.01, ***p<0.001 for comparing tofacitinib 5 mg twice daily versus placebo (up to week 16) or placebo→tofacitinib 5 mg twice daily (up to week 48). P values are reported without adjustment for multiple comparisons. †Patients receiving placebo advanced to tofacitinib 5 mg twice daily at week 16 (dashed line). ∆, change from baseline; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BID, twice daily; LS, least squares; MMRM, mixed model for repeated measures; N, number of patients in full analysis set; N1, number of patients with observation at visit, if different from the full analysis set.