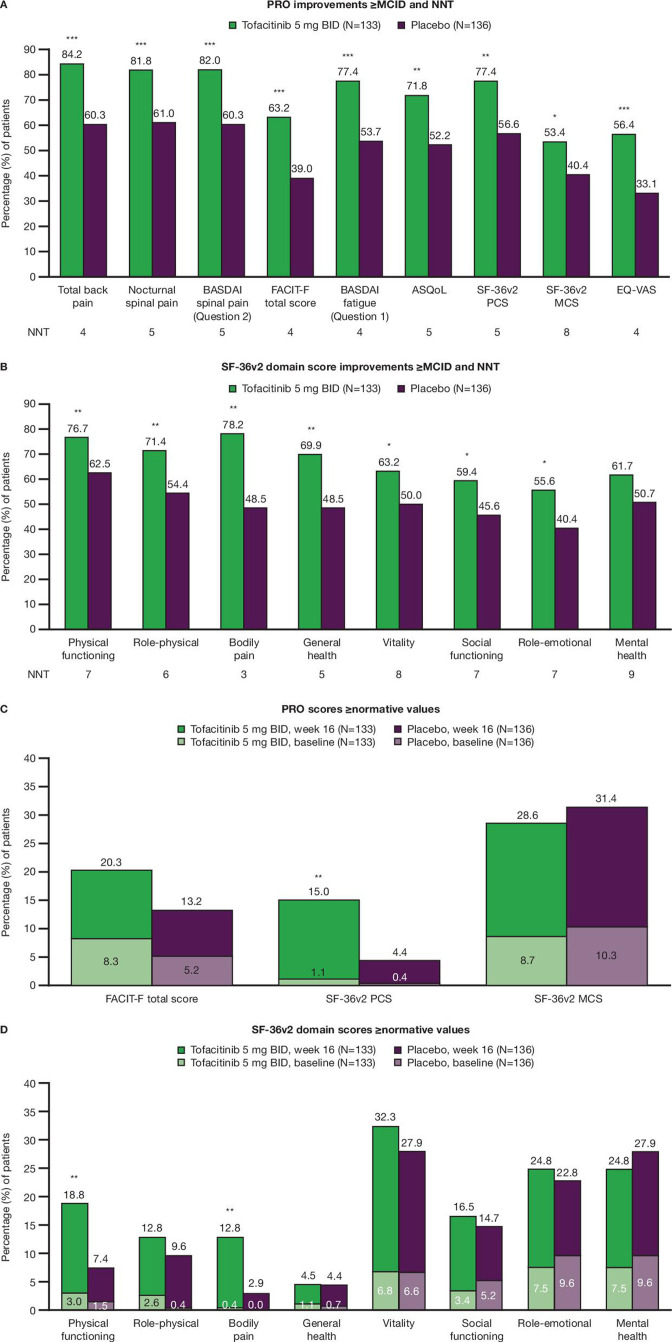
Figure 2.
PRO (A, B) improvements ≥MCID† and NNTs,‡ and (C, D) scores ≥normative values§ at week 16. Data are from the week 16 analysis: data cut-off 19 December 2019; data snapshot 29 January 2020. Missing response was considered as non-response. P values are nominal. *p≤0.05, **p<0.01, ***p<0.001 for comparing tofacitinib 5 mg twice daily versus placebo at week 16; Cochran-Mantel-Haenszel approach adjusting for stratification factor (bDMARD-naïve versus TNFi-IR or bDMARD use (non-IR)). †MCID cut-offs: total back pain, nocturnal spinal pain, BASDAI overall spinal pain and BASDAI fatigue, decrease from baseline ≥1; FACIT-F total score, increase from baseline ≥4.0; ASQoL, decrease from baseline ≥1.8; SF-36v2 PCS and MCS scores, increase from baseline ≥2.5; eight SF-36v2 domain 0–100 scores, increase from baseline ≥5.0: EQ-VAS, increase from baseline ≥10 mm. ‡NNT defined as the inverse of the difference in proportions of patients in the tofacitinib arm versus placebo arm reporting ≥MCID. §≥normative values: FACIT-F total score, ≥43.5; SF-36v2 PCS and MCS scores, ≥50; and eight SF-36v2 domain 0–100 scores: physical functioning, ≥88.23; role-physical, ≥87.96; bodily pain, ≥76.81; general health, ≥73.00; vitality, ≥60.55; social functioning, ≥87.66; role-emotional, ≥91.04; mental health, ≥76.70 (the domain-specific cut-offs were calculated as the study protocol’s age-distributed and sex-distributed means matched to the 1998 US population norms on the raw scale with a range of 0–100). AS, ankylosing spondylitis; ASQoL, Ankylosing Spondylitis Quality of Life; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; bDMARD, biologic disease-modifying antirheumatic drug; BID, twice daily; EQ-VAS, EuroQol Visual Analogue Scale; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; IR, inadequate response; MCID, minimum clinically important difference; MCS, Mental Component Summary; N, number of patients in full analysis set; NNT, number needed to treat; PCS, Physical Component Summary; PRO, patient-reported outcome; SF-36v2, Short Form-36 Health Survey Version 2; TNFi, tumour necrosis factor inhibitor.