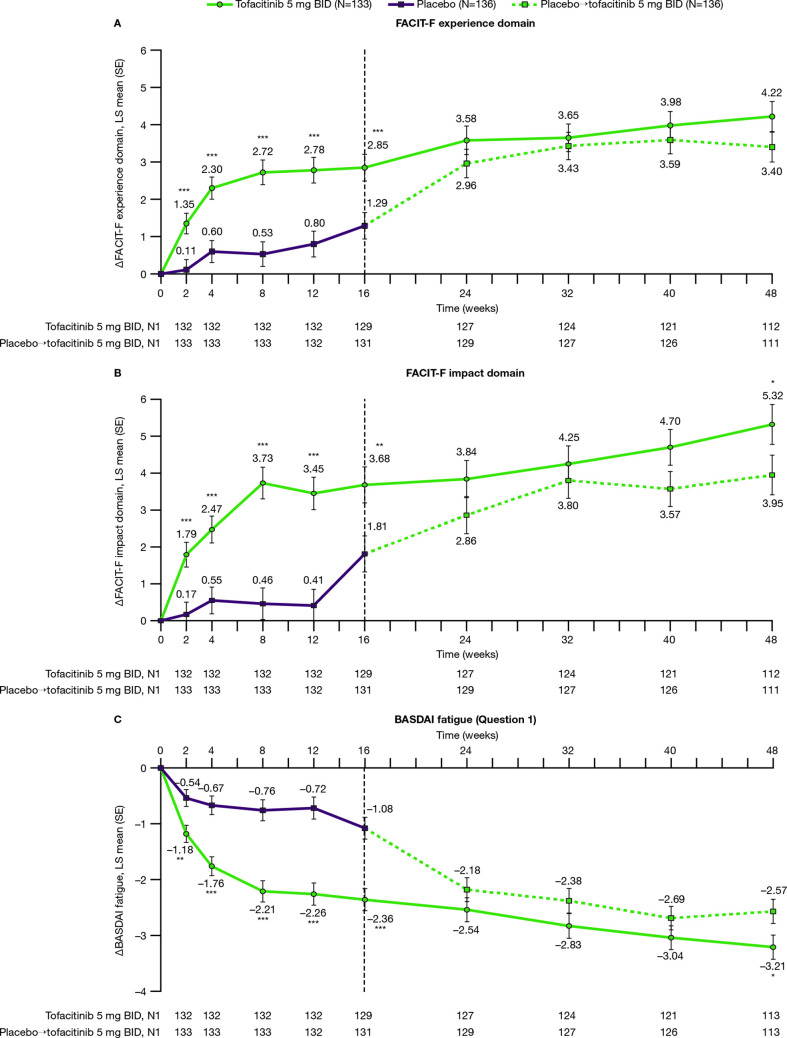
Figure 3.
Changes from baseline to week 48: FACIT-F (A) experience, (B) impact and (C) BASDAI fatigue. LS mean changes from baseline to week 48 for FACIT-F (A) experience, (B) impact and (C) BASDAI fatigue in patients with AS receiving tofacitinib 5 mg twice daily or placebo→tofacitinib 5 mg twice daily.† Results up to week 16, based on MMRM, include all postbaseline data to week 16 (data cut-off 19 December 2019; data snapshot 29 January 2020); results after week 16 are based on another MMRM including all postbaseline data to week 48 (reporting results after week 16 only). *p≤0.05, **p<0.01, ***p<0.001 for comparing tofacitinib 5 mg twice daily versus placebo (up to week 16) or placebo→tofacitinib 5 mg twice daily (up to week 48). P values are reported without adjustment for multiple comparisons. †Patients receiving placebo advanced to tofacitinib 5 mg twice daily at week 16 (dashed line). ∆, change from baseline; AS, ankylosing spondylitis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BID, twice daily; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HRQoL, health-related quality of life; LS, least squares; MMRM, mixed model for repeated measures; N, number of patients in full analysis set; N1, number of patients with observation at visit, if different from the full analysis set.