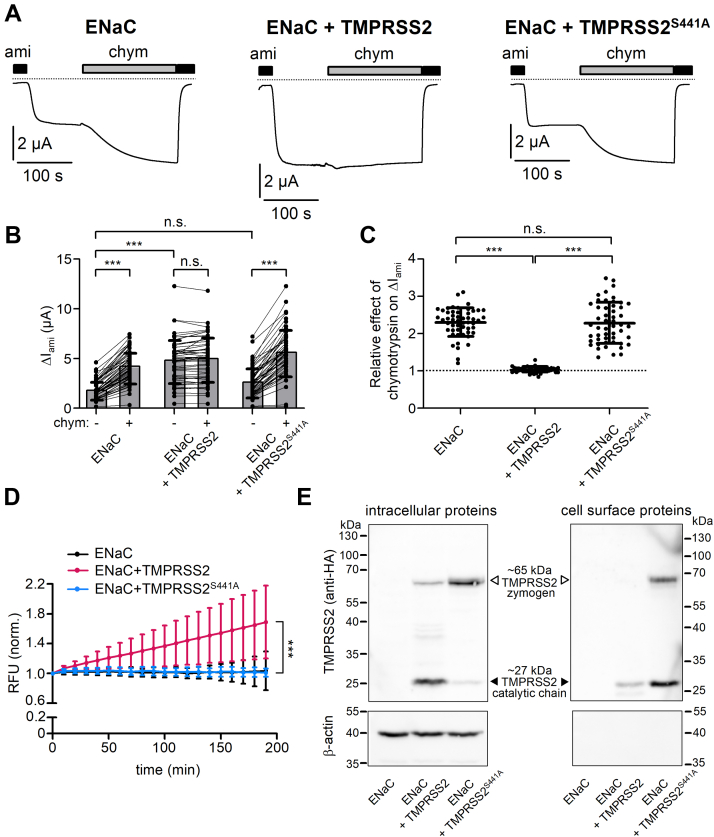
Figure 1.
Stimulatory effect of TMPRSS2 on ENaC requires proteolytic activity of TMPRSS2.A, representative whole-cell current traces are shown for oocytes expressing human wildtype αβγ-ENaC alone (left trace, ENaC) or coexpressing αβγ-ENaC with human wildtype TMPRSS2 (middle trace, ENaC + TMPRSS2) or catalytically inactive TMPRSS2 (right trace, ENaC + TMPRSS2S441A). HA tag epitope was attached to the C terminus of TMPRSS2. HA tag neither disturbed the proteolytic activity of TMPRSS2 nor affected the stimulatory effect of TMPRSS2 on ENaC (Fig. S1). Amiloride (ami, 2 μM) and chymotrypsin (chym, 2 μg/ml) were present in the bath solution as indicated by black and gray bars, respectively. Dashed lines indicate zero current level. B, ENaC-mediated ami-sensitive whole-cell currents (ΔIami) were determined from similar experiments as shown in (A) by subtracting the baseline current in the presence of ami from the current level reached in its absence before (−) or after (+) chym application. Lines connect data points obtained in an individual oocyte. Mean ± SD and data points for individual oocytes are shown; ∗∗∗p < 0.001; ns, Kruskal–Wallis with Dunn’s post hoc test (51 ≤ n ≤ 52, N = 5). C, relative stimulatory effect of chym on ΔIami summarized from data shown in (B). Dashed line indicates normalized ΔIami value of one (no effect). Mean ± SD and data points for individual oocytes are shown; ∗∗∗p < 0.001; ns, one-way ANOVA with Bonferroni post hoc test. D, in parallel experiments to those shown in (A–C), trypsin-like proteolytic activity at the cell surface was detected in the same batches of oocytes. Progress curves of proteolytic activity (RFU = relative fluorescent unit; mean ± SD) are shown. In each individual recording RFU values were normalized to the initial RFU value at the beginning of the measurement. ∗∗∗p < 0.001; Kruskal–Wallis with Dunn’s post hoc test (at the time point 190 min; 41≤ n ≤ 46, N = 5). E, representative western blots showing intracellular (left upper panel) or cell surface (right upper panel) expression of HA-tagged wildtype TMPRSS2 or mutant TMPRSS2S441A in oocytes from one batch. No specific signal was detected with the anti-HA antibody in oocytes expressing ENaC alone. TMPRSS2 zymogen (∼65 kDa) and TMPRSS2 in its activated cleaved form (catalytic chain, ∼27 kDa) are indicated by open and filled arrowheads, respectively. To validate separation of cell surface proteins from intracellular proteins, blots were stripped and reprobed using an antibody against β-actin (lower panels). Similar results were obtained in three additional repeats (n = 4). ENaC, epithelial sodium channel; HA, hemagglutinin; ns, not significant; TMPRSS2, transmembrane serine protease 2.