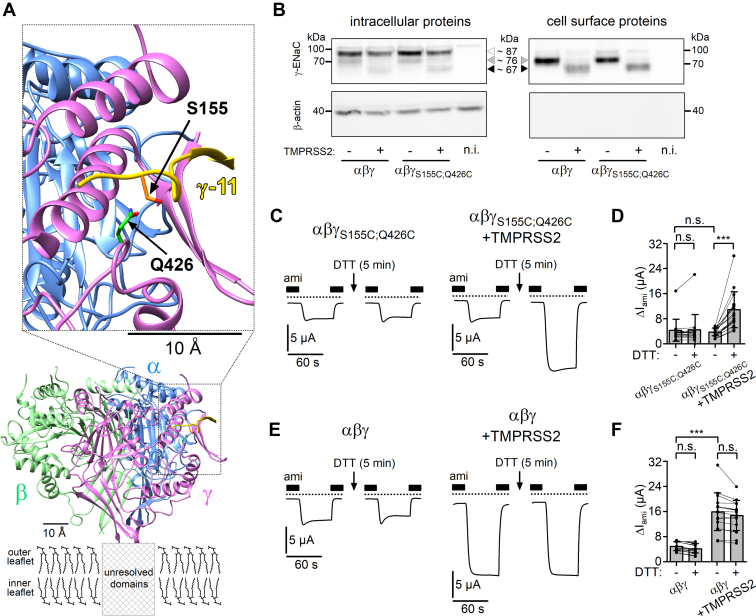
Figure 5.
Preventing the release of the inhibitory tract from γ-ENaC abolishes the stimulatory effect of TMPRSS2 on ENaC.A, ribbon diagram of extracellular domains of human αβγ-ENaC generated using atom coordinates from PDB entry 6WTH (8, 77). The putative location of unresolved transmembrane domains is indicated with a box placed within the plasma lipid bilayer (outer and inner leaflets), which is schematically depicted with dipalmitoylphosphatidylcholine (DPPC) molecules in stick representation. The inset shows the location of the specific binding site of the key inhibitory amino acid sequence (γ-11, in yellow) of the γ-inhibitory tract on an expanded scale. Serine (S155) and glutamine (Q426) residues, which were substituted by cysteines to introduce a disulfide bond between γ-inhibitory tract and its binding site, are indicated with arrows and shown in stick representation with side-chain carbons in orange for S155 or green for Q426, nitrogen in blue, and oxygens in red. Hydrogen atoms are omitted for clarity. B, representative western blots showing intracellular (left upper panel) or cell surface (right upper panel) expression of γ-ENaC in oocytes expressing wildtype (αβγ) or mutant (αβγS155C;Q426C) ENaC without or with TMPRSS2 coexpression. Specific signal of γ-ENaC was detected using the same antibody as for Figure 4. Noninjected oocytes served as control (n.i.). Positions of uncleaved (∼87 kDa), partially cleaved (∼76 kDa), and fully cleaved γ-ENaC (∼67 kDa) are indicated by open, gray, and black-filled arrowheads, respectively. To validate separation of cell surface proteins from intracellular proteins, blots were stripped and reprobed using an antibody against β-actin (lower panels). Similar results were obtained in another repeat (n = 2). C and E, representative whole-cell current traces are shown for oocytes expressing the mutant ENaC (αβγS155C;Q426C, C) or wildtype ENaC (αβγ, E) without (left panels) or with TMPRSS2 coexpression (right panels). In each individual oocyte currents were measured before and after 5 min incubation in ND96 bath solution containing DTT (30 mM) and amiloride (ami; 2 μM). The experimental protocol was similar to that described for Figure 2. The presence of ami (2 μM) is indicated by filled bars. Dashed lines indicate zero current level. D and F, summary of ΔIami values obtained in similar experiments as shown in (C) and (E). Lines connect data points obtained in an individual oocyte. Mean ± SD and data points for individual oocytes are shown; ∗∗∗p < 0.001; ns, Kruskal–Wallis with Dunn’s post hoc test (16≤ n ≤ 18, N = 3). ENaC, epithelial sodium channel; ns, not significant; PDB, Protein Data Bank; TMPRSS2, transmembrane serine protease 2.