Abstract
Antibodies against myelin oligodendrocyte glycoprotein (MOG-IgG) have recently been established as a biomarker for MOG-antibody-associated disease (MOGAD), which is a distinct demyelinating disease of the central nervous system. Among the diverse clinical phenotypes of MOGAD, myelitis is the second-most-common presentation in adults, followed by optic neuritis. While some features overlap, there are multiple reports of distinctive clinical and radiological features of MOG-IgG-associated myelitis, which are useful for differentiating MOGAD from both multiple sclerosis and neuromyelitis optica spectrum disorder. In this review we summarize the clinical and radiographic characteristics of MOG-IgG-associated myelitis with a particular focus on adult patients.
Keywords: myelin oligodendrocyte glycoprotein, demyelinating diseases, myelitis, multiple sclerosis, neuromyelitis optica spectrum disorder
INTRODUCTION
In recent decades, the knowledge of inflammatory demyelinating diseases (IDDs) in the central nervous system (CNS) has expanded markedly with the identification of two novel glial autoantibodies. These advances started with the discovery of antibodies against the astrocytic water channel, aquaporin-4 (AQP4).1,2 This improved the understanding of neuromyelitis optica spectrum disorder (NMOSD), which consists of mainly longitudinally extensive transverse myelitis (LETM) and/or optic neuritis (ON). AQP4 antibodies (AQP4-IgG) are present in more than 70% of patients with NMOSD, and are useful as a critical diagnostic biomarker.3 Approximately a decade later, antibodies against myelin oligodendrocyte glycoprotein (MOG-IgG) were reported in a subset of AQP4-IgG-negative NMOSD patients;4,5 MOG-IgG-positive patients with ON and LETM were classified initially as a subtype of AQP4-IgG-negative NMOSD. However, MOG-antibody-associated disease (MOGAD) shows broad clinical features beyond those observed in NMOSD, including ON, myelitis, acute disseminated encephalomyelitis (ADEM), cortical encephalitis, and other brain syndromes.3 Less than 25% of patients with MOGAD satisfy the current NMOSD criteria, despite its predilection for the optic nerve and spinal cord.6,7 While a relapsing course is typical in most patients with multiple sclerosis (MS) or AQP4-IgG-positive NMOSD, both monophasic and relapsing courses are possible in MOGAD.8 In addition, lesions in MOGAD differ histopathologically from those in MS and AQP4-IgG-positive NMOSD.9 Based on this evidence, MOGAD is recognized as a distinct disease entity from MS and NMOSD.
MOG is expressed on the surface of the myelin sheath, mainly in CNS neurons, and accounts for about 0.5% of myelin components.10,11 Previous attempts to detect MOG-IgG using ELISA or Western blot analysis have produced inconsistent and confusing results due to the low specificity of these techniques.12 However, live cell-based assays (CBAs) using MOG-transfected cells have increased the sensitivity and reliability in detecting MOG-IgG,10,12 and this has played a major role in revealing the distinct clinical and radiological features of MOGAD.
In adult patients with MOGAD, myelitis is the second-most-common presentation, followed by the ON phenotype, while isolated myelitis is an uncommon phenotype in pediatric patients.12,13,14 Due to the diverse etiologies of myelitis, a thorough understanding of the clinical and radiological features of MOG-IgG-associated myelitis (henceforth MOG-IgG myelitis) can assist neurologists in assessing and designing appropriate treatment strategies. This article summarizes the current knowledge on MOG-IgG myelitis with a particular focus on the adult population. Only studies using CBAs for detecting MOG-IgG are included in the review.
EPIDEMIOLOGY OF MOGAD
Among CNS-IDDs, MOG-IgG are detected more frequently in children than in adults. A large cohort study including more than 15,000 subjects from the Mayo Clinic detected MOG-IgG in 6.5% of adult patients with CNS-IDDs, compared with 21.1% of pediatric patients.15 This markedly higher prevalence of MOG-IgG in pediatric CNS-IDDs is consistent with the findings of studies conducted in various countries, where MOG-IgG were found in 22%–31% of pediatric patients with CNS-IDDs,16,17,18 compared with 4%–6% MOG-IgG positivity among adult patients.19,20,21 In addition, the frequency of MOGAD varies according to the clinical phenotype. In cases of ADEM, MOG-IgG are identified in up to 64% of pediatric patients and 60% of adult patients;22,23 in marked contrast, the frequency of MOG-IgG positivity is low (2%–4%) among adult patients with ON.24,25 MOG-IgG tests performed at the time of acute myelopathy found that 6.6% (11/166) of patients were seropositive for MOG-IgG, including both children and adults; however, the results for 55% (6/11) of the MOG-IgG-positive patients were finally determined to be false positives based on the current international diagnostic recommendation.12,26 Further studies are therefore needed into the frequency of MOG-IgG positivity among myelitis cases. Unlike MS and NMOSD, which are characterized by predominantly female patients, most studies have found no significant sex preference in MOGAD.15,27,28 Also, it remains unclear if there is a specific racial predilection in MOGAD.
It is particularly notable that MOG-IgG were found in up to 45% of patients with AQP4-IgG-negative NMOSD diagnosed using the International Panel for NMO Diagnosis 2015 criteria: 45% (9/20) of patients in a Spanish cohort and 42% (15/36) of patients in a UK cohort were seropositive for MOG-IgG.16,29 With regard to AQP4-IgG-negative LETM, 7%–23% patients tested seropositive for MOG-IgG.30,31 In contrast, MOG-IgG are rarely detected in patients with typical MS; 1.5% (24/1,608) of MS patients were reported to be seropositive for MOG-IgG across 25 studies using CBAs with an immunofluorescence method.10 A recent large multicenter study found that only 0.3% (2/685) of MS patients had MOG-IgG.32 Therefore, the routine serological test for MOG-IgG is not recommended in patients with typical MS,12 and the probability of false-positive results should be considered when interpreting low-titer positivity of MOG-IgG in cases of typical MS.
Overall, the most common phenotype in adults with MOGAD is ON, accounting for about half of cases: 56% (150/268) and 53% (103/193) of adults with MOGAD presented with isolated ON in French and UK studies, respectively.27,33 In addition, the clinical presentation of MOGAD is clearly associated with the age at onset. ADEM is the most common presentation in young children with MOGAD, while an opticospinal phenotype (ON and/or myelitis) is more common in adults. The phenotypic conversion from ADEM to opticospinal presentation has a bimodal distribution with a cutoff around 9 years of age.10 Adult patients generally have a higher risk of relapse throughout the disease course, and display a lower tendency for full recovery from an attack, as compared with pediatric patients.27,33
Myelitis is the second-most-common presentation in adults with MOGAD, reportedly accounting for 18%–52% of cases, and the proportion increases up to 59% during the entire disease course.19,27,28,34,35,36,37,38,39 More than half of myelitis attacks occur in an isolated form, and the remaining show a mixed phenotype that includes ON and/or brain involvement.19,34,35,39,40 When MOG-IgG myelitis does not occur alone, it is mostly accompanied by ON.40,41 Isolated myelitis is a relatively unusual phenotype in pediatric patients with MOGAD, and literature focusing on pediatric MOG-IgG myelitis is sparse. Thus, this review focuses on adult patients with MOG-IgG myelitis.
CLINICAL FEATURES OF MOG-IgG MYELITIS
Clinical manifestation of acute myelitis
Similar to myelitis with other etiologies, clinical manifestations of MOG-IgG myelitis include motor weakness, sensory deficit, and sphincter dysfunction.40,41,42 Motor and sensory symptoms are observed in 70%–90% of patients;40,42 these frequencies are the same as those in AQP4-IgG myelitis.41 The severity of motor deficits in patients with MOG-IgG myelitis varies at nadir, but it is usually moderate or severe and comparable with that for AQP4-IgG myelitis.41 Nationwide multicenter studies from France found that patients with an Expanded Disability Status Scale (EDSS) score of ≥3.0 accounted for 89.4% of adult cases,40 and 56% (15/27) of adults with MOG-IgG myelitis displayed an EDSS score of ≥6.0 at the time of onset.33 Other studies from Spain, UK, and China also showed high median EDSS scores (5.5, 4.0, and 3.0, respectively) at the nadir of MOG-IgG myelitis.37,41,43 A US study found that the proportion of patients with MOG-IgG myelitis who needed a cane or walker was 41%, and one-third were dependent on a wheelchair at nadir.42 The severity of the motor deficit appears to depend on the length of the spinal lesion; motor impairment was more severe in patients with LETM (more than two vertebral segments) than in those with short-segment myelitis (up to two vertebral segments).40
Sphincter dysfunction can be a more predominant feature of MOG-IgG myelitis, compared with MS or NMOSD, and is found in 66%–83% of cases.40,41,42,43 Bladder and/or bowel dysfunction were reported to occur more frequently in MOG-IgG myelitis than in MS myelitis (83% vs. 31%, p<0.011)42 and AQP4-IgG myelitis (68% vs. 43%, p=0.011).43 In addition, erectile dysfunction was observed in about half of males with MOG-IgG myelitis.27,42
MOG-IgG myelitis causes sensory symptoms such as paresthesia and hypoesthesia, but neuropathic pain tends to occur less often in MOG-IgG myelitis than in AQP4-IgG myelitis. A comparative study reported that neuropathic pain at onset was observed in 13% of patients with MOG-IgG myelitis, which was less common than in AQP4-IgG myelitis patients (29%, p=0.04).41 In other studies focused on the pain characteristics of each disease, 23% (8/34) of patients with MOG-IgG myelitis suffered from neuropathic pain,44 which was observed in 62% (31/50) of patients with NMOSD.45 In addition, painful tonic spasm (PTS), which has been reported in 22%–43% of patients with NMOSD,46,47,48 was not found in previous MOGAD studies. One recent study found that PTS was markedly more common in AQP4-IgG myelitis than in MOG-IgG myelitis (47% vs. 11%, p<0.001).43 Thus, severe neuropathic pain and PTS may be more suggestive of AQP4-IgG myelitis than MOG-IgG myelitis.
Relapse risk of MOG-IgG myelitis
MOG-IgG myelitis tends to relapse more frequently in adults than in children. Relapses after a first myelitis episode were reported in 37% (17/46) of MOG-IgG myelitis cases, with a mean interval of 31 months from onset to relapse.41 In a multicenter French cohort study, 35% of adults with MOG-IgG myelitis who had at least one episode of myelitis throughout the disease course had a relapse;40 this relapse rate is higher than that of pediatric patients (0%–14%).49,50,51 In a similar context, adults with MOGAD whose initial manifestation was myelitis had a 2.01-fold higher risk of relapse throughout the disease course than did children, according to a Cox regression model.33
Relapse following a myelitis attack is usually not confined to the spinal cord in MOGAD, whereas about one-third of patients with AQP4-IgG myelitis have disease restricted to the spinal cord throughout the disease course.52 Mariano et al.41 reported that only 12% (2/17) of patients with MOG-IgG myelitis experienced a further relapse with an isolated myelitis phenotype. More than half of relapse cases present with ON after a myelitis episode.41,42 In addition, another study found that the mean annualized relapse rate was lower in patients with LETM than in those with short-segment myelitis (0.13 vs. 0.35, p=0.001).40
It is especially important that many relapses occur at the time of steroid withdrawal or tapering in MOGAD.7,14 Because the early cessation or rapid taper of steroid treatment can lead to a disease flare-up, the duration of steroid treatment is an important consideration. Experts generally suggest administering oral steroid for 3–6 months in adult patients with MOGAD.7
Recovery from an acute attack and the clinical outcome
Most patients with MOG-IgG myelitis show good motor recovery from acute myelitis episodes. In contrast to moderate or severe motor deficits at the peak of the disease, the last median EDSS score has been lower than 2.5 in most studies, suggesting minimal disability.40,41,43,53 Complete recovery was observed in 25%–27% of patients with MOG-IgG myelitis.40,54 In terms of the acute treatment response, 92% (48/52) of patients with MOG-IgG myelitis experienced disability improvement after acute treatments that included at least one of high-dose intravenous methylprednisolone, plasmapheresis, and intravenous immunoglobulin.42 Compared with patients with AQP4-IgG myelitis, the clinical outcome measured by the EDSS score at last follow-up was significantly better.41,43,53 Severe disability can remain in a minority of MOG-IgG myelitis cases, but it is much less common than in AQP4-IgG myelitis. One study found that the proportion of patients needing a walking aid at the last follow-up was 6% regardless of whether or not further relapses developed; another study found that severe disability (EDSS score ≥6) after a single myelitis episode remained in 7% of patients, which was much less common than in AQP4-IgG myelitis patients (44%).41,42 In our experience, severe motor deficits rarely remain after a single attack of MOG-IgG myelitis.
It is also notable that sphincter dysfunction commonly lingers in MOG-IgG myelitis patients. Long-term sphincter symptoms can be a predominant disability despite good recovery from motor deficits. Bladder or bowel dysfunction remains in 28%–59% of patients with MOG-IgG myelitis, and about one-fifth of patients require long-term urinary catheterization.27,41,42 Mariano et al.41 reported that long-term catheterization was associated with the presence of a conus lesion but not with the EDSS score at the last follow-up, which suggests that the EDSS score—which mainly focuses on motor disability—does not fully reflect the clinical outcome in cases of MOG-IgG myelitis. Thus, a new tool for evaluating clinical outcomes in MOG-IgG myelitis that emphasizes residual sphincter dysfunction is required, since this markedly affects the quality of life.55
MRI FINDINGS IN MOG-IgG MYELITIS
Lesion characteristics and contrast enhancement
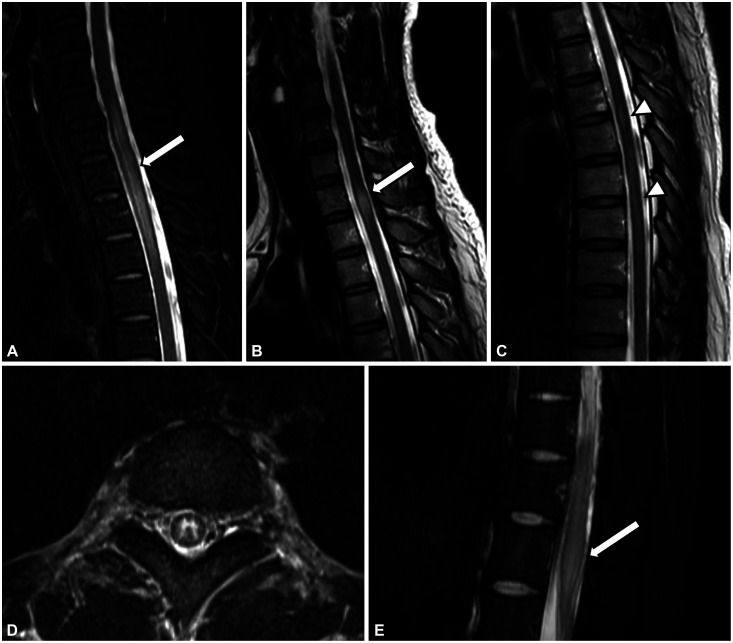
Similar to AQP4-IgG myelitis, LETM is most commonly observed in patients with MOG-IgG myelitis (40%–80%).14,28,36,40,41,42,43 However, short-segment cord lesions are also frequently seen in MOGAD; Mariano et al.41 noted that 39% patients had multiple spinal cord lesions and about half had short-segment lesions, and Jarius et al.14 reported that 44% of all myelitis attacks with MOG-IgG were associated with a non-LETM pattern. Therefore, LETM is a dominant pattern in MOG-IgG myelitis, but short-segment lesions are also common (Fig. 1A-C).
Fig. 1. Spine MRI findings in MOG-IgG myelitis. A-C: Patterns of spinal lesion length in MOG-IgG myelitis. Sagittal T2-weighted images showing (A) an LETM pattern, (B) a non-LETM pattern, and (C) a mixed pattern. Cord swelling is commonly present at the acute stage of MOG-IgG myelitis (white arrows in A and B). High signal intensities restricted to the gray matter on axial sequences forming the H-sign (D) and conus involvement (E, white arrow) are frequently detected in MOG-IgG myelitis. LETM, longitudinally extensive transverse myelitis; MOG-IgG, myelin oligodendrocyte glycoprotein antibodies.
Axial T2-weighted lesions reportedly appear in the central portion of the spinal cord in 75%–83% of cases, and more than 50% of the axial area is usually covered.41,56,57 A high signal intensity, sometimes restricted to the gray matter on axial sequences, can form the “H-sign” (Fig. 1D). This is found in 29%–50% of MOG-IgG myelitis cases, which is more frequent than in AQP4-IgG myelitis (8%).42,43 In cases of MS myelitis, the H-sign is extremely rare and is considered a red flag.42
Spinal cord MRI lesions can involve the entire spinal cord in MOG-IgG myelitis, but conus medullaris involvement suggests MOGAD rather than MS or NMOSD (Fig. 1E). Conus lesions were detected in 21%–41% cases of MOG-IgG myelitis, which is a higher prevalence than in AQP4-IgG myelitis (2%–13%) and MS myelitis (1%–33%).40,41,42,43,57,58 A recent investigation of the diagnostic value of conus lesions found that affected patients had a 13-fold higher probability of being diagnosed with MOGAD than did those without a conus lesion.58 This conus involvement may be associated with a high frequency of sphincter dysfunction in MOG-IgG myelitis patients.41 In addition, several MOG-IgG myelitis cases with lumbosacral root thickening and enhancements have been reported, usually accompanied by conus lesions.59,60,61
Spinal cord swelling is a common MRI finding (60%–100% of cases) at the acute stage of MOG-IgG myelitis (Fig. 1A and B).14,57,62 Given that most patients with MOG-IgG myelitis experience a good recovery from acute myelitis attacks, spinal cord edema is considered transient and reversible. The prevalence of lesions with contrast enhancement reportedly varies widely (26%–80%), and they generally present with patchy and heterogeneous features with blurred margins.14,41,42,57,63 A comparison of different CNS-IDDs revealed that the rate of contrast enhancement at the acute stage is lower for MOG-IgG myelitis (54%) than for MS (89%) and NMOSD (94%).63 In addition, persistent enhancement at follow-up or signs of necrosis are rare in MOG-IgG myelitis.14,63
The bright spotty lesion (BSL) is a distinctive radiological feature of AQP4-IgG myelitis that has been detected in 27%–54% of cases.64,65,66,67 In contrast, this feature is rarely found in myelitis with other etiologies; for example, BSL was evident in only 3%–6% of patients with AQP4-IgG-negative myelitis such as MS or idiopathic myelitis.65,66 The reported incidence of BSL in MOG-IgG myelitis has differed among studies: two small studies found that 50% (2/4 or 5/10) of cases with MOG-IgG myelitis had BSLs, which were simply defined as bright T2-weighted hyperintensities.62,68 In contrast, a study that used the original BSL definition—“very hyperintense spotty lesions on T2-weighted imaging that are visually more hyperintense or of equal signal intensity to the surrounding cerebrospinal fluid (CSF) without flow void effects”—detected no BSL in MOG-IgG myelitis cases.66 We recently directly compared the incidence of BSL between AQP4-IgG and MOG-IgG myelitis using the refined terminology of “brighter spotty lesion” in order to avoid misinterpreting BSLs by emphasizing that a hyperintense lesion in T2-weighted imaging is brighter than a usual T2-weighted hyperintense lesion.67 Our study found that none of 49 MOG-IgG myelitis patients showed BSL, while 30% (18/61) of patients with AQP4-IgG myelitis had BSL in the acute phase.67 It appears that BSL is rarely seen in MOG-IgG myelitis and is more representative of AQP4-IgG myelitis, suggesting that applying an appropriate BSL definition can be useful for discriminating between AQP4-IgG and MOG-IgG myelitis.
Invisible MRI lesions have been reported in the acute stage of MOG-IgG myelitis.14,69,70 Jarius et al.14 reported that spinal lesions were not found in 2 attacks of 27 patients with MOG-IgG myelitis, and Sechi et al.70 reported that definite spinal lesions in initial spine MRI were not detected in 10% of cases (7/70), despite the presence of clear myelitis symptoms. Thus, MRI-negative myelitis is an uncommon but possible manifestation of MOG-IgG myelitis. Measuring somatosensory evoked potentials or performing follow-up MRI may help to support the presence of or detect initially invisible lesions.
Lesions resolution and cord atrophy
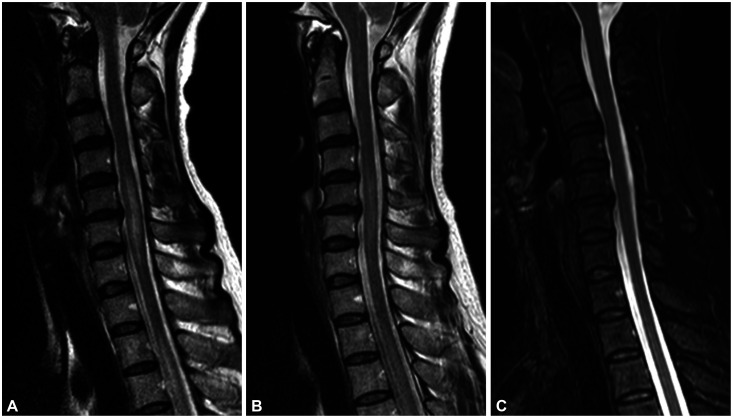
Spine MRI lesions in MOG-IgG myelitis tend to resolve completely at follow-up despite their largeness and the presence of severe disability during acute attacks, which is consistent with the good recovery reported in patients with MOG-IgG myelitis. Sechi et al.63 demonstrated that the sizes of T2-weighted lesions in attack MRI are similar for MOG-IgG and AQP4-IgG myelitis, and that these lesions are significantly larger than those in MS myelitis. In follow-up MRI, MOG-IgG myelitis shows a larger reduction in axial lesion size than that observed in both AQP4-IgG and MS myelitis, with median size reductions of T2-weighted lesions relative to the attack MRI of 100%, 71%, and 53%, respectively. It is especially notable that the complete resolution of acute T2-weighted lesions in follow-up MRI has been reported in 62%–84% of MOG-IgG myelitis cases (Fig. 2). In contrast, spinal lesions in NMOSD and MS rarely resolve completely, with small residual T2-weighted lesions usually remaining.41,53,57,63 Compared with AQP4-IgG and MS myelitis, lesions in MOG-IgG myelitis are characterized by a large initial lesion, a significant size reduction of the lesion at follow-up, and often complete resolution.
Fig. 2. MRI images showing resolution of the signal abnormality in MOG-IgG myelitis. Changes in spinal cord lesions in a 49-year-old female with MOG-IgG myelitis. A: Cervical LETM (C4–7) with cord swelling detected in initial MRI. B: Significant reduction in signal abnormalities and swelling after 2 weeks. C: Complete resolution of acute T2-weighted lesions without significant cord atrophy after 2 months. LETM, longitudinally extensive transverse myelitis; MOG-IgG, myelin oligodendrocyte glycoprotein antibodies.
Spinal cord atrophy after a myelitis attack is uncommon in MOG-IgG myelitis.42,68 Dubey et al.42 reported that only 3% (1/35) of patients had residual cord atrophy at the last follow-up. A study that analyzed changes in cross-sectional area (CSA) in follow-up MRI as measured at least 6 months after an acute event found no significant decrease in the CSA of the spinal cord in MOG-IgG myelitis compared with that either in the presence of previous lesions or in healthy volunteers; whereas the CSA was significantly smaller in AQP4-IgG or MS myelitis than in healthy controls.68 It was particularly interesting that MOG-IgG myelitis patients with a cervical cord lesion exhibited a selective decrease in the gray-matter volume but no significant changes in the entire CSA, when compared with MOG-IgG myelitis without a cervical cord lesion.
Table 1 summarizes the MRI characteristics of three CNS-IDDs: MOG-IgG, AQP4-IgG myelitis, and MS myelitis. The common MRI findings of MOG-IgG myelitis are presented in Figs. 1 and 2.
Table 1. Comparison of spine MRI features in MS, AQP4-IgG, and MOG-IgG myelitis.
| Myelitis | MOG-IgG | AQP4-IgG | MS |
|---|---|---|---|
| Sagittal extension | Commonly LETM (40%–80%) Short-segment myelitis may be present (–48%) | Usually LETM Infrequent short-segment myelitis | Usually short-segment myelitis Extremely rare LETM |
| Number of lesions | Single or multiple | Usually single | Commonly multiple |
| Location | Anywhere in the spinal cord Frequent conus involvement (21%–41%) | Cervical and/or thoracic cord Infrequent conus involvement | Cervical > thoracic cord Infrequent conus involvement |
| Axial involvement | Central cord preference H-sign in 29%–50% | Central cord preference H-sign may be seen (–10%) | Peripheral cord preference and partial involvement Extremely rare H-sign |
| Cord swelling and atrophy | Frequent cord swelling at acute stage Rare cord atrophy | Frequent cord swelling at acute stage (often longitudinal cord swelling) Regional cord atrophy may be present | Infrequent cord swelling (focal if present) Diffuse cord atrophy may be present at later stage |
| BSLs | Extremely rare | Present in 27–54% | Extremely rare |
| Complete lesion resolution | Common (62%–84%) | Uncommon | Uncommon |
| Contrast enhancement | Variable (26%–80%) Leptomeningeal enhancement may be present | Usually present | Usually present |
AQP4-IgG, aquaporin-4 antibodies; BSL, bright spotty lesion; LETM, longitudinally extensive transverse myelitis; MOG-IgG, myelin oligodendrocyte glycoprotein antibodies; MS, multiple sclerosis.
LABORATORY FINDINGS IN MOG-IgG MYELITIS
CSF pleocytosis is commonly observed in MOG-IgG myelitis, with a level exceeding 50 cells/mm3 observed in about 50% of patients,40,42,71 which is significantly higher than that in MS myelitis.42 In addition, CSF protein levels are commonly elevated in MOG-IgG myelitis patients. Sechi et al.71 reported that the median concentration of CSF protein was 64 mg/dL in 35 patients with MOG-IgG myelitis, and that 77% of them showed an elevated protein level (>45 mg/dL). Compared with other attack phenotypes of MOGAD, CSF pleocytosis and elevated protein levels are more common in MOG-IgG myelitis than in the ON phenotype, but these elevations may be comparable with those in the brain phenotype.6,71 The preceding treatment can influence the CSF findings, such as patients who were treated with corticosteroids before a CSF examination showing lower frequencies of both pleocytosis and CSF protein elevation.71 The extent of the spinal lesion may affect the CSF profiles. In the case of LETM, the degree of CSF pleocytosis and protein levels tend to be even higher. CSF pleocytosis was more frequently observed in LETM patients (77.3%) than in non-LETM patients (48.1%).40 One small study found remarkable pleocytosis (mean: 218 cells/mm3, range 49–353 cells/mm3) and high protein levels (mean: 465 mg/dL, range 64–1100 mg/dL) in the CSF of six patients with LETM.72
The frequency of CSF-specific oligoclonal bands (OCBs) has been reported to range from 3% to 20% in patients with MOG-IgG myelitis, which suggests that OCB positivity cannot exclude the possibility of MOG-IgG myelitis.6,33,37,40,41,42,54,71 However, OCBs were found in only 0%–5% of MOGAD patients with an isolated ON phenotype.33,54,71 Thus, in cases with an isolated ON phenotype, the presence of OCBs would be suggestive of MS than MOGAD considering the high prevalence (about 90%) of OCBs in MS.73,74
RED FLAGS IN MOG-IgG MYELITIS
Red flags and suggestive features of MOG-IgG myelitis are listed in Table 2. Because most patients show good motor recovery from acute myelitis attacks, severe motor disability rarely occurs after a single attack of MOG-IgG myelitis. Therefore, if severe paraplegia remains after a single episode, a myelopathy etiology other than MOG-IgG should be suspected. In addition, it usually takes from one to several days to reach the symptomatic peak in inflammatory demyelinating myelopathy, including MOG-IgG myelitis. When the time to the nadir deficit is less than a few hours, other etiologies such as spinal cord infarction should be considered.75 It should be noted that a progressive course is not characteristic of MOG-IgG myelitis. Several studies have found that MOG-IgG are either not or only rarely detected in patients with progressive MS.32,76 There is a recent report of five cases of progressive myelopathy having MOG-IgG,77 but they had MRI findings suggestive of MS, including short-segment myelitis and periventricular lesions, and three of them had low titers of MOG-IgG, and so the possibility of false-positive results for MOG-IgG cannot be excluded. Based on the published literature and our own experience, a progressive course is exceptionally rare in MOG-IgG myelitis. Spinal cord atrophy after a myelitis attack, BSLs, and persistent enhancements can also be considered as radiological red flags (Table 2).
Table 2. Red flags and suggestive features in MOG-IgG myelitis.
| Clinical red flags | Suggestive clinical manifestations |
|---|---|
| 1. Residual severe paraplegia after a single myelitis attack | 1. Good motor recovery |
| 2. Rapid severe deficit (≤4 h to nadir deficit) | 2. Predominant residual sphincter dysfunction |
| 3. Slowly progressive myelopathy in the absence of an attack | |
| Radiological red flags | Suggestive radiological findings |
| 1. Cord atrophy after a single attack | 1. Conus involvement |
| 2. Presence of “brighter spotty lesion” | 2. Central cord preference and formation of the H-sign |
| 3. Persistent enhancement | 3. Complete lesion resolution at follow-up |
MOG-IgG, myelin oligodendrocyte glycoprotein antibodies.
CONCLUSION
This article has summarized how knowledge of the clinical and radiological features in MOG-IgG myelitis has increased over the past few years. Although myelitis can occur in all CNS-IDDs and some overlaps may exist, MOG-IgG myelitis exhibits specific clinical and MRI characteristics that help to differentiate it from AQP4-IgG and MS myelitis. Recognition of these distinctive features in MOG-IgG myelitis can assist the differential diagnosis and help guide appropriate treatment options.
Footnotes
- Conceptualization: Ho Jin Kim.
- Resources: all authors.
- Supervision: Su-Hyun Kim, Ho Jin Kim.
- Visualization: Ki Hoon Kim.
- Writing—original draft: Ki Hoon Kim.
- Writing—review & editing: all authors.
Conflicts of Interest: KH Kim reports no disclosures.
SH Kim has lectured, consulted, and received honoraria from Bayer Schering Pharma, Biogen, Genzyme, Merck Serono, and UCB and received a grant from the National Research Foundation of Korea. JW Hyun has received a grant from the National Research Foundation of Korea.
HJ Kim received a grant from the National Research Foundation of Korea and research support from Aprilbio and Eisai; received consultancy/speaker fees from Alexion, Aprilbio, Biogen, Celltrion, Daewoong, Eisai, GC Pharma, HanAll BioPharma, Horizon Therapeutics (foremly Viela Bio), MDimune, Merck Serono, Novartis, Roche, Sanofi Genzyme, Teva-Handok, and UCB; is a co-editor for the Multiple Sclerosis Journal and an associate editor for the Journal of Clinical Neurology.
Funding Statement: This work was supported by the National Research Foundation of Korea (Grant No. NRF-2018R1A5A2023127).
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
References
- 1.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 2.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79:1273–1277. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 5.Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Haidar Jorge FM, Takahashi T, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82:474–481. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. 2018;90:e1858–e1869. doi: 10.1212/WNL.0000000000005560. [DOI] [PubMed] [Google Scholar]
- 7.Ramanathan S, Mohammad S, Tantsis E, Nguyen TK, Merheb V, Fung VSC, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89:127–137. doi: 10.1136/jnnp-2017-316880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marignier R, Hacohen Y, Cobo-Calvo A, Pröbstel AK, Aktas O, Alexopoulos H, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20:762–772. doi: 10.1016/S1474-4422(21)00218-0. [DOI] [PubMed] [Google Scholar]
- 9.Takai Y, Misu T, Kaneko K, Chihara N, Narikawa K, Tsuchida S, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain. 2020;143:1431–1446. doi: 10.1093/brain/awaa102. [DOI] [PubMed] [Google Scholar]
- 10.Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15:89–102. doi: 10.1038/s41582-018-0112-x. [DOI] [PubMed] [Google Scholar]
- 11.Pham-Dinh D, Mattei MG, Nussbaum JL, Roussel G, Pontarotti P, Roeckel N, et al. Myelin/oligodendrocyte glycoprotein is a member of a subset of the immunoglobulin superfamily encoded within the major histocompatibility complex. Proc Natl Acad Sci U S A. 1993;90:7990–7994. doi: 10.1073/pnas.90.17.7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15:134. doi: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. 2020;6:85. doi: 10.1038/s41572-020-0214-9. [DOI] [PubMed] [Google Scholar]
- 14.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13:280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunchok A, Chen JJ, McKeon A, Mills JR, Flanagan EP, Pittock SJ. Coexistence of myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies in adult and pediatric patients. JAMA Neurol. 2020;77:257–259. doi: 10.1001/jamaneurol.2019.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sepúlveda M, Aldea M, Escudero D, Llufriu S, Arrambide G, Otero-Romero S, et al. Epidemiology of NMOSD in Catalonia: influence of the new 2015 criteria in incidence and prevalence estimates. Mult Scler. 2018;24:1843–1851. doi: 10.1177/1352458517735191. [DOI] [PubMed] [Google Scholar]
- 17.de Mol CL, Wong YYM, van Pelt ED, Ketelslegers IA, Bakker DP, Boon M, et al. Incidence and outcome of acquired demyelinating syndromes in Dutch children: update of a nationwide and prospective study. J Neurol. 2018;265:1310–1319. doi: 10.1007/s00415-018-8835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hennes EM, Baumann M, Schanda K, Anlar B, Bajer-Kornek B, Blaschek A, et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89:900–908. doi: 10.1212/WNL.0000000000004312. [DOI] [PubMed] [Google Scholar]
- 19.Hyun JW, Lee HL, Jeong WK, Lee HJ, Shin JH, Min JH, et al. Comparison of MOG and AQP4 antibody seroprevalence in Korean adults with inflammatory demyelinating CNS diseases. Mult Scler. 2021;27:964–967. doi: 10.1177/1352458520948213. [DOI] [PubMed] [Google Scholar]
- 20.Hyun JW, Woodhall MR, Kim SH, Jeong IH, Kong B, Kim G, et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry. 2017;88:811–817. doi: 10.1136/jnnp-2017-315998. [DOI] [PubMed] [Google Scholar]
- 21.Kim SM, Woodhall MR, Kim JS, Kim SJ, Park KS, Vincent A, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm. 2015;2:e163. doi: 10.1212/NXI.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Chiriboga AS, Majed M, Fryer J, Dubey D, McKeon A, Flanagan EP, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG–associated disorders. JAMA Neurol. 2018;75:1355–1363. doi: 10.1001/jamaneurol.2018.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossor T, Benetou C, Wright S, Duignan S, Lascelles K, Robinson R, et al. Early predictors of epilepsy and subsequent relapse in children with acute disseminated encephalomyelitis. Mult Scler. 2020;26:333–342. doi: 10.1177/1352458518823486. [DOI] [PubMed] [Google Scholar]
- 24.Soelberg K, Jarius S, Skejoe H, Engberg H, Mehlsen JJ, Nilsson AC, et al. A population-based prospective study of optic neuritis. Mult Scler. 2017;23:1893–1901. doi: 10.1177/1352458517734070. [DOI] [PubMed] [Google Scholar]
- 25.Chen JJ, Tobin WO, Majed M, Jitprapaikulsan J, Fryer JP, Leavitt JA, et al. Prevalence of myelin oligodendrocyte glycoprotein and aquaporin-4–IgG in patients in the Optic Neuritis Treatment Trial. JAMA Ophthalmol. 2018;136:419–422. doi: 10.1001/jamaophthalmol.2017.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sechi E, Buciuc M, Pittock SJ, Chen JJ, Fryer JP, Jenkins SM, et al. Positive predictive value of myelin oligodendrocyte glycoprotein autoantibody testing. JAMA Neurol. 2021;78:741–746. doi: 10.1001/jamaneurol.2021.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128–3138. doi: 10.1093/brain/awx276. [DOI] [PubMed] [Google Scholar]
- 28.de Mol CL, Wong Y, van Pelt ED, Wokke B, Siepman T, Neuteboom RF, et al. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler. 2020;26:806–814. doi: 10.1177/1352458519845112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamid SHM, Whittam D, Mutch K, Linaker S, Solomon T, Das K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017;264:2088–2094. doi: 10.1007/s00415-017-8596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyun JW, Kim SH, Huh SY, Kim W, Yun J, Joung A, et al. Idiopathic aquaporin-4 antibody negative longitudinally extensive transverse myelitis. Mult Scler. 2015;21:710–717. doi: 10.1177/1352458514551454. [DOI] [PubMed] [Google Scholar]
- 31.Cobo-Calvo Á, Sepúlveda M, Bernard-Valnet R, Ruiz A, Brassat D, Martínez-Yélamos S, et al. Antibodies to myelin oligodendrocyte glycoprotein in aquaporin 4 antibody seronegative longitudinally extensive transverse myelitis: clinical and prognostic implications. Mult Scler. 2016;22:312–319. doi: 10.1177/1352458515591071. [DOI] [PubMed] [Google Scholar]
- 32.Cobo-Calvo Á, d’Indy H, Ruiz A, Collongues N, Kremer L, Durand-Dubief F, et al. Frequency of myelin oligodendrocyte glycoprotein antibody in multiple sclerosis: a multicenter cross-sectional study. Neurol Neuroimmunol Neuroinflamm. 2019;7:e649. doi: 10.1212/NXI.0000000000000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobo-Calvo A, Ruiz A, Rollot F, Arrambide G, Deschamps R, Maillart E, et al. Clinical features and risk of relapse in children and adults with myelin oligodendrocyte glycoprotein antibody-associated disease. Ann Neurol. 2021;89:30–41. doi: 10.1002/ana.25909. [DOI] [PubMed] [Google Scholar]
- 34.Alshamrani F, Alnajashi H, Shosha E, Casserly C, Morrow SA. Case series: myelin oligodendrocyte glycoprotein-immunoglobulin G-related disease spectrum. Front Neurol. 2020;11:89. doi: 10.3389/fneur.2020.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cobo-Calvo A, Sepúlveda M, Rollot F, Armangué T, Ruiz A, Maillart E, et al. Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J Neuroinflammation. 2019;16:134. doi: 10.1186/s12974-019-1525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Mori M, Zimmermann H, Brandt A, Havla J, Tanaka S, et al. Anti-MOG antibody-associated disorders: differences in clinical profiles and prognosis in Japan and Germany. J Neurol Neurosurg Psychiatry. 2020 Nov 20; doi: 10.1136/jnnp-2020-324422. [Epub]. Available from: [DOI] [PubMed] [Google Scholar]
- 37.Sepúlveda M, Armangue T, Martinez-Hernandez E, Arrambide G, Sola-Valls N, Sabater L, et al. Clinical spectrum associated with MOG autoimmunity in adults: significance of sharing rodent MOG epitopes. J Neurol. 2016;263:1349–1360. doi: 10.1007/s00415-016-8147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Netravathi M, Holla VV, Nalini A, Yadav R, Vengalil S, Oommen AT, et al. Myelin oligodendrocyte glycoprotein-antibody-associated disorder: a new inflammatory CNS demyelinating disorder. J Neurol. 2021;268:1419–1433. doi: 10.1007/s00415-020-10300-z. [DOI] [PubMed] [Google Scholar]
- 39.Cobo-Calvo Á, Ruiz A, D’Indy H, Poulat AL, Carneiro M, Philippe N, et al. MOG antibody-related disorders: common features and uncommon presentations. J Neurol. 2017;264:1945–1955. doi: 10.1007/s00415-017-8583-z. [DOI] [PubMed] [Google Scholar]
- 40.Ciron J, Cobo-Calvo A, Audoin B, Bourre B, Brassat D, Cohen M, et al. Frequency and characteristics of short versus longitudinally extensive myelitis in adults with MOG antibodies: a retrospective multicentric study. Mult Scler. 2020;26:936–944. doi: 10.1177/1352458519849511. [DOI] [PubMed] [Google Scholar]
- 41.Mariano R, Messina S, Kumar K, Kuker W, Leite MI, Palace J. Comparison of clinical outcomes of transverse myelitis among adults with myelin oligodendrocyte glycoprotein antibody vs aquaporin-4 antibody disease. JAMA Netw Open. 2019;2:e1912732. doi: 10.1001/jamanetworkopen.2019.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubey D, Pittock SJ, Krecke KN, Morris PP, Sechi E, Zalewski NL, et al. Clinical, radiologic, and prognostic features of myelitis associated with myelin oligodendrocyte glycoprotein autoantibody. JAMA Neurol. 2019;76:301–309. doi: 10.1001/jamaneurol.2018.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ZhangBao J, Huang W, Zhou L, Wang L, Chang X, Lu C, et al. Myelitis in inflammatory disorders associated with myelin oligodendrocyte glycoprotein antibody and aquaporin-4 antibody: a comparative study in Chinese Han patients. Eur J Neurol. 2021;28:1308–1315. doi: 10.1111/ene.14654. [DOI] [PubMed] [Google Scholar]
- 44.Asseyer S, Henke E, Trebst C, Hümmert MW, Wildemann B, Jarius S, et al. Pain, depression, and quality of life in adults with MOG-antibody-associated disease. Eur J Neurol. 2021;28:1645–1658. doi: 10.1111/ene.14729. [DOI] [PubMed] [Google Scholar]
- 45.Zhao S, Mutch K, Elsone L, Nurmikko T, Jacob A. Neuropathic pain in neuromyelitis optica affects activities of daily living and quality of life. Mult Scler. 2014;20:1658–1661. doi: 10.1177/1352458514522103. [DOI] [PubMed] [Google Scholar]
- 46.Kim SM, Go MJ, Sung JJ, Park KS, Lee KW. Painful tonic spasm in neuromyelitis optica: incidence, diagnostic utility, and clinical characteristics. Arch Neurol. 2012;69:1026–1031. doi: 10.1001/archneurol.2012.112. [DOI] [PubMed] [Google Scholar]
- 47.Li QY, Wang B, Yang J, Zhou L, Bao JZ, Wang L, et al. Painful tonic spasm in Chinese patients with neuromyelitis optica spectrum disorder: prevalence, subtype, and features. Mult Scler Relat Disord. 2020;45:102408. doi: 10.1016/j.msard.2020.102408. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Zhang Q, Lian Z, Chen H, Shi Z, Feng H, et al. Painful tonic spasm in neuromyelitis optica spectrum disorders: prevalence, clinical implications and treatment options. Mult Scler Relat Disord. 2017;17:99–102. doi: 10.1016/j.msard.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Waters P, Fadda G, Woodhall M, O’Mahony J, Brown RA, Castro DA, et al. Serial anti-myelin oligodendrocyte glycoprotein antibody analyses and outcomes in children with demyelinating syndromes. JAMA Neurol. 2020;77:82–93. doi: 10.1001/jamaneurol.2019.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duignan S, Wright S, Rossor T, Cazabon J, Gilmour K, Ciccarelli O, et al. Myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies are highly specific in children with acquired demyelinating syndromes. Dev Med Child Neurol. 2018;60:958–962. doi: 10.1111/dmcn.13703. [DOI] [PubMed] [Google Scholar]
- 51.Lechner C, Baumann M, Hennes EM, Schanda K, Marquard K, Karenfort M, et al. Antibodies to MOG and AQP4 in children with neuromyelitis optica and limited forms of the disease. J Neurol Neurosurg Psychiatry. 2016;87:897–905. doi: 10.1136/jnnp-2015-311743. [DOI] [PubMed] [Google Scholar]
- 52.Kitley J, Leite MI, Nakashima I, Waters P, McNeillis B, Brown R, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain. 2012;135(Pt 6):1834–1849. doi: 10.1093/brain/aws109. [DOI] [PubMed] [Google Scholar]
- 53.Höftberger R, Sepulveda M, Armangue T, Blanco Y, Rostásy K, Calvo AC, et al. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler. 2015;21:866–874. doi: 10.1177/1352458514555785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y, Jia X, Yang H, Chen C, Sun X, Peng L, et al. Myelin oligodendrocyte glycoprotein antibody-associated demyelination: comparison between onset phenotypes. Eur J Neurol. 2019;26:175–183. doi: 10.1111/ene.13791. [DOI] [PubMed] [Google Scholar]
- 55.Beekman J, Keisler A, Pedraza O, Haramura M, Gianella-Borradori A, Katz E, et al. Neuromyelitis optica spectrum disorder: patient experience and quality of life. Neurol Neuroimmunol Neuroinflamm. 2019;6:e580. doi: 10.1212/NXI.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mariano R, Messina S, Roca-Fernandez A, Leite MI, Kong Y, Palace JA. Quantitative spinal cord MRI in MOG-antibody disease, neuromyelitis optica and multiple sclerosis. Brain. 2021;144:198–212. doi: 10.1093/brain/awaa347. [DOI] [PubMed] [Google Scholar]
- 57.Kitley J, Waters P, Woodhall M, Leite MI, Murchison A, George J, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. 2014;71:276–283. doi: 10.1001/jamaneurol.2013.5857. [DOI] [PubMed] [Google Scholar]
- 58.Etemadifar M, Salari M, Kargaran PK, Sigari AA, Nouri H, Etemadifar F, et al. Conus medullaris involvement in demyelinating disorders of the CNS: a comparative study. Mult Scler Relat Disord. 2021;54:103127. doi: 10.1016/j.msard.2021.103127. [DOI] [PubMed] [Google Scholar]
- 59.Vazquez Do Campo R, Stephens A, Marin Collazo IV, Rubin DI. MOG antibodies in combined central and peripheral demyelination syndromes. Neurol Neuroimmunol Neuroinflamm. 2018;5:e503. doi: 10.1212/NXI.0000000000000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tantsis EM, Prelog K, Alper G, Benson L, Gorman M, Lim M, et al. Magnetic resonance imaging in enterovirus-71, myelin oligodendrocyte glycoprotein antibody, aquaporin-4 antibody, and multiple sclerosis-associated myelitis in children. Dev Med Child Neurol. 2019;61:1108–1116. doi: 10.1111/dmcn.14114. [DOI] [PubMed] [Google Scholar]
- 61.Sundaram S, Nair SS, Jaganmohan D, Unnikrishnan G, Nair M. Relapsing lumbosacral myeloradiculitis: an unusual presentation of MOG antibody disease. Mult Scler. 2020;26:509–511. doi: 10.1177/1352458519840747. [DOI] [PubMed] [Google Scholar]
- 62.Salama S, Khan M, Levy M, Izbudak I. Radiological characteristics of myelin oligodendrocyte glycoprotein antibody disease. Mult Scler Relat Disord. 2019;29:15–22. doi: 10.1016/j.msard.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sechi E, Krecke KN, Messina SA, Buciuc M, Pittock SJ, Chen JJ, et al. Comparison of MRI lesion evolution in different central nervous system demyelinating disorders. Neurology. 2021;97:e1097–e1109. doi: 10.1212/WNL.0000000000012467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hyun JW, Kim SH, Jeong IH, Lee SH, Kim HJ. Bright spotty lesions on the spinal cord: an additional MRI indicator of neuromyelitis optica spectrum disorder? J Neurol Neurosurg Psychiatry. 2015;86:1280–1282. doi: 10.1136/jnnp-2014-309761. [DOI] [PubMed] [Google Scholar]
- 65.Yonezu T, Ito S, Mori M, Ogawa Y, Makino T, Uzawa A, et al. “Bright spotty lesions” on spinal magnetic resonance imaging differentiate neuromyelitis optica from multiple sclerosis. Mult Scler. 2014;20:331–337. doi: 10.1177/1352458513495581. [DOI] [PubMed] [Google Scholar]
- 66.Rabasté S, Cobo-Calvo A, Nistiriuc-Muntean V, Vukusic S, Marignier R, Cotton F OFSEP, NOMADMUS Study Group. Diagnostic value of bright spotty lesions on MRI after a first episode of acute myelopathy. J Neuroradiol. 2021;48:28–36. doi: 10.1016/j.neurad.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Hyun JW, Lee HL, Park J, Kim J, Min JH, Kim BJ, et al. Brighter spotty lesions on spinal MRI help differentiate AQP4 antibody-positive NMOSD from MOGAD. Mult Scler. 2021 Dec 06; doi: 10.1177/13524585211060326. [Epub]. Available from: [DOI] [PubMed] [Google Scholar]
- 68.Chien C, Scheel M, Schmitz-Hübsch T, Borisow N, Ruprecht K, Bellmann-Strobl J, et al. Spinal cord lesions and atrophy in NMOSD with AQP4-IgG and MOG-IgG associated autoimmunity. Mult Scler. 2019;25:1926–1936. doi: 10.1177/1352458518815596. [DOI] [PubMed] [Google Scholar]
- 69.Macaron G, Ontaneda D. MOG-related disorders: a new cause of imaging-negative myelitis? Mult Scler. 2020;26:511–515. doi: 10.1177/1352458519840746. [DOI] [PubMed] [Google Scholar]
- 70.Sechi E, Krecke KN, Pittock SJ, Dubey D, Lopez-Chiriboga AS, Kunchok A, et al. Frequency and characteristics of MRI-negative myelitis associated with MOG autoantibodies. Mult Scler. 2021;27:303–308. doi: 10.1177/1352458520907900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sechi E, Buciuc M, Flanagan EP, Pittock SJ, Banks SA, Lopez-Chiriboga AS, et al. Variability of cerebrospinal fluid findings by attack phenotype in myelin oligodendrocyte glycoprotein-IgG-associated disorder. Mult Scler Relat Disord. 2021;47:102638. doi: 10.1016/j.msard.2020.102638. [DOI] [PubMed] [Google Scholar]
- 72.Loos J, Pfeuffer S, Pape K, Ruck T, Luessi F, Spreer A, et al. MOG encephalomyelitis: distinct clinical, MRI and CSF features in patients with longitudinal extensive transverse myelitis as first clinical presentation. J Neurol. 2020;267:1632–1642. doi: 10.1007/s00415-020-09755-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dobson R, Ramagopalan S, Davis A, Giovannoni G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry. 2013;84:909–914. doi: 10.1136/jnnp-2012-304695. [DOI] [PubMed] [Google Scholar]
- 74.Kim KH, Kim SH, Park NY, Hyun JW, Kim HJ. Reappraisal of CSF-specific oligoclonal bands in Asia. Mult Scler. 2022;28:665–668. doi: 10.1177/13524585211048752. [DOI] [PubMed] [Google Scholar]
- 75.Zalewski NL, Rabinstein AA, Krecke KN, Brown RD, Jr, Wijdicks EFM, Weinshenker BG, et al. Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurol. 2019;76:56–63. doi: 10.1001/jamaneurol.2018.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jarius S, Ruprecht K, Stellmann JP, Huss A, Ayzenberg I, Willing A, et al. MOG-IgG in primary and secondary chronic progressive multiple sclerosis: a multicenter study of 200 patients and review of the literature. J Neuroinflammation. 2018;15:88. doi: 10.1186/s12974-018-1108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marcucci SB, Elkasaby M, Walch R, Zare-Shahabadi A, Mahammedi A, Abboud H, et al. Progressive myelopathy in myelin oligodendrocyte glycoprotein antibody-associated disease: a new mimicker of progressive multiple sclerosis? Mult Scler Relat Disord. 2021;52:102964. doi: 10.1016/j.msard.2021.102964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.