Abstract
Objective
This study aims to compare the accuracy of CT‐based preoperative planning with that of acetate templating in predicting implant size, neck length, and neck cut length, and to evaluate the reproducibility of the two methods.
Methods
This prospective study was conducted between August 2020 and March 2021. Patients who underwent elective primary total hip arthroplasty by a single surgeon were assessed for eligibility. The included patients underwent both acetate templating and CT‐based planning by two observers after the operation. Each observer conducted both acetate templating and CT‐based planning twice for each case. The outcome measures included the following: (1) the accuracy of surgical planning in predicting implant size, calcar length, and neck length, which was defined as the difference between the planned size and length and the actual size and length; (2) reproducibility of the two planning techniques, which were assessed by inter‐observer and intra‐observer reliability analysis; (3) the influence of potential confounding factors on planning accuracy, which was evaluated using generalized estimating equations.
Results
A total of 57 cases were included in the study. CT‐based planning was more accurate than acetate templating for predicting cup size (93% vs 79%, p < 0.001) and stem size (93% vs 75%, p < 0.001). When assessed by mean absolute difference, the comparison between acetate templating and CT‐based planning was 4.28 mm vs 3.74 mm (p = 0.122) in predicting neck length and 3.05 mm vs 2.93 mm (p = 0.731) in predicting neck cut length. In the inter‐observer reliability analysis, an intraclass correlation coefficient (ICC) of 0.790 was achieved for predicting cup size, and an ICC of 0.966 was achieved for predicting stem size using CT‐based planning. In terms of intra‐observer reliability, Observer 1 achieved an ICC of 0.803 for predicting cup size and 0.965 for predicting stem size in CT‐based planning. Observer 2 achieved ICC values of 0.727 and 0.959 for predicting cup and stem sizes, respectively. The average planning time was 6.48 ± 1.55 min for CT‐based planning and 6.12 ± 1.40 min for acetate templating (p = 0.015).
Conclusion
The CT‐based planning system is more accurate than acetate templating for predicting implant size and has good reproducibility in total hip arthroplasty.
Keywords: arthroplasty, computer tomography, hip, preoperative planning, X‐ray
A prospective study was conducted to investigate the accuracy and reproducibility of acetate templating and CT‐based planning for THA. The accuracy of CT‐based planning (over 90%) is higher than acetate templating (over 70%). Both methods showed satisfactory reproducibility. Planner experience, BMI, and surgical experience did not influence planning reliability.
Introduction
The need for total hip arthroplasty (THA) has been increasing rapidly over the past few decades, and the annual surgery volume was estimated to reach 600,000 by 2030 in the United States 1 . In addition to the alleviation of pain, prolonged survival and satisfactory functional outcomes have become equally important 2 . To achieve these goals, a high level of precision is required to choose the appropriate size of implants and place them in correct positions, which helps minimize leg length discrepancy (LLD), restore offset, and avoid complications, including aseptic loosening and periprosthetic fracture 3 . Preoperative planning helps determine implant position and size, adjustment of leg length, and adjustment of offset in total hip arthroplasty. Accurate and reproducible preoperative planning is an essential and integral part of a successful total hip arthroplasty 4 . In addition, accurate planning increases efficiency in the operating room and avoids excessive inventory stock of implants. 5
Preoperative planning can be performed using radiography and computed tomography (CT). Acetate templating remains the primary option for preoperative planning for many surgeons 6 . The accuracy of acetate templating in predicting implant size varies widely in the literature (60%–99.2%) 2 , 3 , 6 , 7 , 8 , 9 , 10 , 11 . The variable accuracy of X‐ray‐based planning suggests that many factors could potentially influence this planning method. Variations in the magnification rate and projection during X‐ray examination and variation in patient anatomy can influence the accuracy of assessment of radiographs. The measurement of femoral offset on X‐ray has been proven to be inaccurate due to anatomical variations in femoral anteversion among patients 12 . The anterior and posterior walls of the acetabular, femoral anteversion, and intramedullary anatomy of the femur are usually not well‐appreciated in a two‐dimensional image 13 .
CT‐based preoperative planning has been reported to achieve excellent accuracy (>90%) in predicting implant size 13 . CT‐based planning offers three‐dimensional information and allows for accurate assessment of patients' individual anatomy 13 . In a long‐term follow‐up study, Torstein et al. 14 found that preoperative CT planning restored anatomical hip geometry, which might provide sustained clinical improvements with a low complication rate. However, CT‐based planning is associated with additional radiation exposure and cost.
One study compared acetate templating and three‐dimensional CT planning in predicting acetabular cup size and found that acetate templating significantly overpredicted cup size when compared with the CT‐based method 15 . The X‐rays were taken at a 115% magnification rate, which might influence the accuracy of templating 16 . Other studies, although without comparing with CT‐based planning, have shown that acetate templating can accurately predict implant size in as many as 99.2% of the cases 9 .
There is still limited evidence whether acetate templating can achieve an accuracy comparable to that of CT‐based planning. Considering the extra radiation exposure and increased cost of CT, X‐ray‐based planning may be the ideal preoperative planning method if it provides equivalent accuracy. It is also unclear which factors might influence the planning accuracy of both planning methods. Planner experience has been shown to influence the accuracy of acetate templating 17 . The influence of the planner experience on CT‐based planning has not yet been investigated.
Previous studies have reported the reproducibility of X‐ray‐based preoperative planning and CT‐based planning. X‐ray‐based planning has shown moderate inter‐observer reliability as assessed by intraclass correlation coefficients (ICC) 6 . In CT‐based planning, Wako et al. 18 reported the inter‐observer reliabilities for predicting component size were above 0.9 for stem size and cup size. However, the inter‐ and intra‐observer reliabilities of acetate templating and CT‐based planning in the same cohort remain uninvestigated.
Therefore, this study aims to: (1) compare the accuracy of CT‐based preoperative planning with that of acetate templating in predicting the actual implant size, neck length, and neck cut length; (2) evaluate the reproducibility and potential factors that influence the accuracy of the two methods.
Material and Methods
Study Design
This study was approved by the hospital's institutional review board (ethics number JS‐2550). This prospective study was conducted between August 2020 and March 2021. Patients were included if: (1) they were 18–85 years old and underwent elective primary total hip arthroplasty; (2) THA was performed by a single surgeon; (3) the patient received both X‐ray and CT examinations of the hip for the comparison of different planning methods; (4) the operation records containing information about the prosthesis used and intraoperative findings were available; (5) postoperative radiographs were available. Patients were excluded if: (1) the radiographs and CT scans were not standardized. A total of 60 cases were assessed, and 57 cases were included in this study. Three cases were excluded due to unstandardized radiographs.
Both acetate templating and CT‐based planning were conducted on patients after surgery by a third‐year orthopaedic resident (Observer 1) and a fifth‐year orthopaedic resident (Observer 2). Each observer conducted acetate templating and CT‐based planning twice for each case at least 4 weeks after the operation. To avoid any bias caused by recollection, each observer was blinded to the patient's identifiable information, and each plan was conducted with an interval of at least 24 hours.
CT‐Based Three‐Dimensional Planning
A CT scan was conducted for each patient according to the following protocol: the scan range was from the highest point of the pelvis to 15 cm below the lesser trochanter with cuts at 1‐mm intervals. Artificial metal reduction CT was performed if the patients had internal fixation of the lower limb.
Three‐dimensional planning was performed using AIHIP software (Version 3.0, Changmugu, Beijing, China). Within the software, artificial intelligence was used for automatic processing of CT images. The process included segmentation of the femur and pelvis and identification of featured anatomic landmarks. Bone landmarks, including the anterior superior iliac crest (ASIS), pubic symphysis, lesser trochanter, and femoral head center were identified. The sagittal pelvic tilt was assessed according to the ASIS‐pubic symphysis plane (APP plane). The femoral offset, leg length discrepancy, neck shaft angle, and other relevant data were calculated. Leg length discrepancy was determined by the distance between the tip of the lesser trochanter and the inter‐ischial line. The results produced by the AI algorithms were manually checked by trained engineers and planners before preoperative planning to ensure accuracy.
Acetabular component: The cup inclination was set at 40° ± 10° and the anteversion of the cup was set at 15° ± 10°. In most cases, the ilio‐ischial line defines the medial border of the cup, and the inter‐teardrop line defines the inferior border of the cup. Size is determined by the position and bone coverage of the cup. At least 70% bone coverage should be achieved. Bone coverage and the amount of bone to be removed can be visualized, and the cup size, inclination, anteversion, and position were adjusted (Figure 1).
Fig. 1.
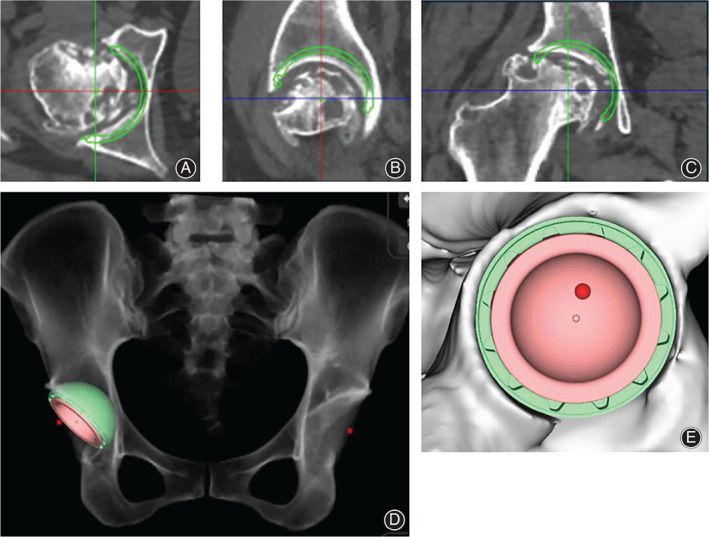
CT‐based planning for acetabular component. (A) Simulation of acetabular component in axial view; (B) Simulation of acetabular component in sagittal view; (C) Simulation of acetabular component in coronal view; (D) Simulation of postoperative X‐ray; (E) Simulation of peripheral cup coverage. The green circle indicates where the stem is implanted in simulation. The red dot indicates the position of original hip center. In this case, the cup was planned at 20° anteversion, 45° inclination, and the cup coverage was 92%.
Femoral component: The principle of femoral component positioning was the same as that described by Gonzalez Della Valle et al. 4 , with additional consideration of the three‐dimensional structure of the medullary cavity. Neck length and neck cut length were measured to facilitate intraoperative assessment (Figure 2).
Fig. 2.
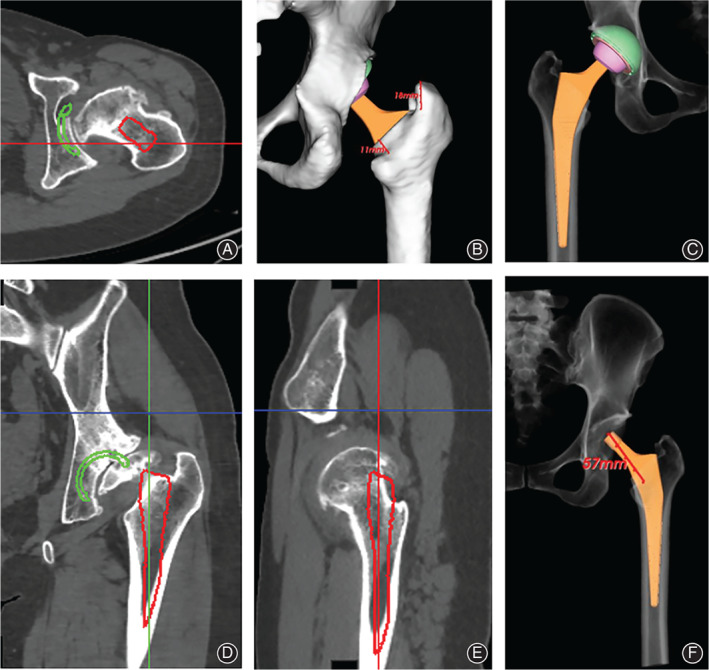
CT‐based planning for femoral component. (A) Simulation of femoral component in axial view; (B) Planned neck cut length and shoulder length; (C) Simulation of postoperative X‐ray; (D) Simulation of femoral component in coronal view; (E) Simulation of femoral component in sagittal view; (F) planned neck length. The red circle indicates where the stem is implanted in simulation.
Acetate Templating
Standard supine pelvic radiographs were acquired with 10° to 15° internal rotation of the hips. The magnification rate was set at 100%. Acetate templating was performed according to the principles and methods described by Gonzalez Della Valle et al. 4 . In general, acetate templating was performed in four steps: preoperative assessment, planning the acetabular component, planning the femoral component and femoral head, and reviewing and adjusting the plan. Preoperative X‐ray measurements were conducted, including the LLD and offset. The position and size of the acetabular component were planned according to the following criteria: On the vertical axis, the bottom of the acetabular component should be placed at the level of the inter‐teardrop line. On the horizontal axis, the acetabular component should approximate the ilio‐ischial line to ensure adequate coverage. The acetabular component was placed at 40° ± 10° of abduction. The position and orientation of the femoral component were planned next, according to the following criteria: the femoral component should be placed in a neutral orientation along the anatomical axis of the femur. The stem size was chosen such that the stem was in contact with the cortical bone medially and laterally. The size and depth of the femoral stem were determined to maintain cortical bone contact, while minimizing LLD and offset changes. The size of the femoral head was chosen to further optimize the LLD and offset. Finally, the surgical plan was reviewed and adjusted as needed to correct any inappropriate prosthesis placement during the previous steps.
Surgical Technique
All operations were performed by a single surgeon in our institution. This surgeon's annual surgical volume for THA is above 100. A standard surgical protocol was followed. In brief, trial components were implanted after femur and acetabular preparations. Reduction was conducted using trial components. The surgeon tested for range of motion, leg length discrepancy, and impingement. Adjustments were made based on the tests after trial reduction. The neck length was measured using the implanted trial prosthesis. Neck length served as a reference for femoral prosthesis implantation. The prosthesis was then implanted. Neck length was measured and compared with that of the trial prosthesis. All patients received cementless prostheses. The acetabular components were Pinnacle (DePuy Orthopedics, Warsaw, IN, USA). The femoral components were the Corail Stem (DePuy Orthopedics, Warsaw, IN, USA) and Trilock Stem (DePuy Orthopedics, Warsaw, IN, USA). Standard perioperative treatment and patient education were provided.
Accurate Prediction of Component Size
The predicted component size was considered correct if it was the same size as the implanted component, or if the difference was within one size.
Neck and Neck Cut Lengths
The neck length was defined as the distance from the femoral rotation center to the tip of the lesser trochanter, and the neck cut length was defined as the distance from the tip of the lesser trochanter to the level of femoral resection. Both measurements were taken intraoperatively using calipers and during surgical planning. The differences between the actual and planned lengths for both neck and neck cut measurements indicate the accuracy of the preoperative plan.
Intra‐ and Inter‐Observer Reliability
Intra‐observer and inter‐observer reliabilities were used to assess planning reproducibility. Intra‐observer reliability refers to the reproducibility of an individual's measurements in two or more observations. Inter‐observer reliability refers to the level of agreement in measurements between different observers.
Intraclass Correlation Coefficient (ICC)
The intraclass correlation coefficient (ICC) was used to assess the intra‐ and inter‐observer reliabilities. ICC describes the agreement of multiple measurements made of one item. An ICC less than 0.20 indicates slight agreement, 0.21–0.40 indicates fair agreement, 0.41–0.60 indicates moderate agreement, 0.61–0.80 indicates substantial agreement, and more than 0.80 indicates almost perfect agreement 20 .
Statistical Analysis
Statistical analyses were performed using SPSS version 25 (IBM, New York, NY, USA) and GraphPad Prism version 8 (GraphPad Software, CA, USA). Continuous variables were recorded as mean ± standard error. Categorical variables were recorded as counts and percentages. The chi‐square test was used to assess the accuracy of preoperative planning. The absolute error was defined as the absolute difference between the planned component size and implanted component size. Generalized estimating equations (GEE) were used to compare the absolute error between the two methods while assessing the influence of confounding factors, including observer experience, BMI, and surgical approach. Statistical significance was set at p < 0.05.
Results
Study Population
Fifty‐seven cases who met the inclusion criteria were prospectively enrolled in this study. Three cases were excluded because of non‐standard radiography. The primary disease was osteonecrosis in 49 cases, developmental dysplasia of the hip (DDH Crowe I) in seven cases, and rheumatoid arthritis in one case. There were 23 men and 34 women. Twenty‐five cases received THA using the direct anterior approach, and 32 cases underwent THA using the posterior approach. The mean age of included patients was 43.64 ± 15.63 years and the mean BMI was 22.07 ± 3.42 kg/m2.
Accuracy of Acetate Templating and CT‐Based Planning
The predicted acetabular component size was exactly the same as the implanted size in 46.49% of the patients in the acetate templating group compared with 52.63% of the patients in the CT‐based planning group (X2 = 1.719, p = 0.190). The predicted acetabular component size was ±1 size from the implanted size in 78.51% of patients in the acetate templating group and 92.98% of patients in the CT‐based planning group (X2 = 19.539, p < 0.001) (Figure 3). The predicted femoral component size was exactly the same as the implanted size in 40.79% of acetate templating cases and 54.82% of CT‐based planning cases (X2 = 9.000, p = 0.003). The femoral component size was accurately predicted in 75% of acetate templating cases compared with 92.54% in CT‐based planning cases (X2 = 25.810, p < 0.001) (Figure 4). The preoperative planning results are listed in Table 1.
Fig. 3.
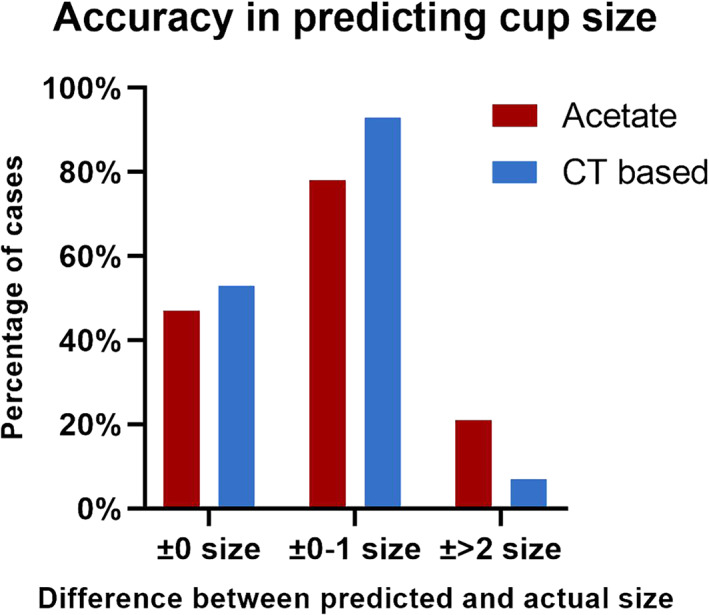
Accuracy of predicting cup size. The figure shows the percentage of the two methods in predicting cup size. The level of accuracy was classified into three categories: ±0 size means the planned size and the actual size was exactly the same; ±0–1 size means the difference between planned size and the actual size was within 1; ±2 size means the difference between planned and the actual size was at least 2.
Fig. 4.
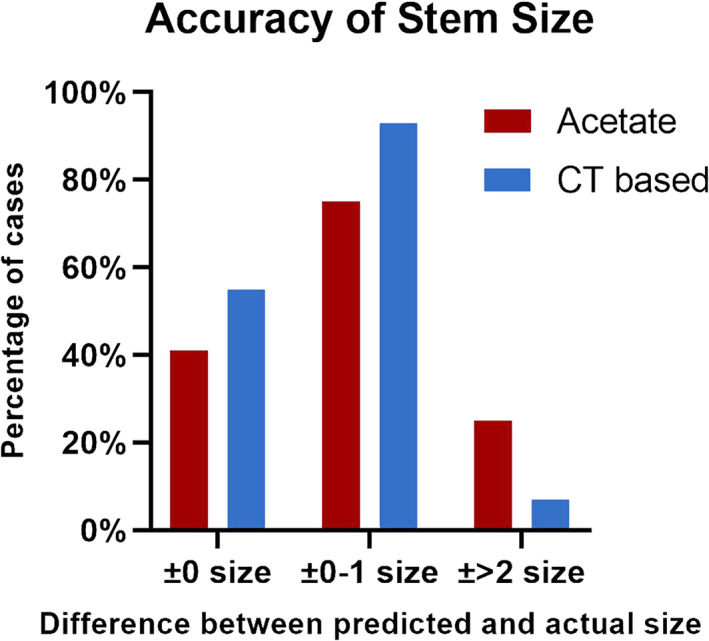
Accuracy of predicting stem size. The figure shows the percentage of the two methods in predicting stem size. The level of accuracy was classified into three categories: ±0 size means the planned size and the actual size was exactly the same; ±0–1 size means the difference between planned size and the actual size was within 1; ±2 size means the difference between the planned and the actual size was at least 2.
TABLE 1.
Difference between predicted component size and actual implanted size by both planning methods
| Cup size | Stem size | |||||
|---|---|---|---|---|---|---|
| Methods | ±0 size | ±0–1 size | ±2+ size | ±0 size | ±0–1 size | ±2+ size |
| Acetate | 46.49% | 78.51% | 21.49% | 40.79% | 75% | 25% |
| CT based | 52.63% | 92.98% | 7.02% | 54.82% | 92.54% | 7.46% |
| X2 Value | 1.719 | 19.539 | 19.539 | 9.000 | 25.810 | 25.810 |
| P Value | 0.19 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 |
The absolute mean error was assessed using the GEE. The mean absolute error of the acetabular component size was 1.588 ± 1.734 in the acetate templating group and 0.991 ± 1.263 in the CT‐based planning group (p < 0.001). The mean absolute error of femoral component size was 0.956 ± 1.038 in the acetate templating group and 0.526 ± 0.631 in the CT‐based planning group (p = 0.001). Observer, surgical approach, diagnosis, and patient BMI were not factors that significantly influenced the agreement between the planned implant size and actual implant size in acetate planning or CT‐based planning. The results are summarized in Table 2.
TABLE 2.
Mean absolute difference between planning and actual implant size and measurement
| Acetate | CT‐based | ||||
|---|---|---|---|---|---|
| Mean absolute error | Mean absolute error | GEE | |||
| Mean | Std. | Mean | Std. | p value | |
| Acetabular component | 1.588 | 1.734 | 0.991 | 1.263 | <0.001 |
| Femoral component | 0.956 | 1.038 | 0.526 | 0.631 | 0.001 |
| Neck length (mm) | 4.275 | 3.379 | 3.740 | 4.547 | 0.122 |
| Neck cut length (mm) | 3.052 | 2.334 | 2.934 | 2.685 | 0.731 |
Std. Standard deviation.
Reproducibility of Acetate Templating and CT‐Based Planning
Intra‐observer reliability was measured using the ICC, which showed substantial intra‐observer agreement in predicting cup size and almost perfect intra‐observer agreement in predicting femoral stem size for both observers. In the prediction of neck length using acetate templating, both observers showed substantial intra‐observer agreement. However, in CT‐based planning, Observer 1 showed an ICC of 0.384 for neck length, indicating fair intra‐observer agreement. Substantial intra‐observer agreement was found when predicting the neck cut length by both observers. Satisfactory inter‐observer agreement was achieved in predicting implant size by both acetate templating and CT‐based planning. The ICC for predicting neck length was 0.450 for acetate templating and 0.543 for CT‐based planning, indicating fair inter‐observer agreement. Substantial inter‐observer agreement was found when predicting neck cut length using both acetate templating and CT‐based planning. The intra‐and inter‐observer reliability results are listed in Table 3.
TABLE 3.
Intra‐observer reliability and inter‐observer reliability
| Intra‐observer reliability (ICC) | |||||
|---|---|---|---|---|---|
| Methods | Observer | Cup | Femoral stem | Neck length | Calcar |
| Acetate | Ob1 | 0.761 | 0.934 | 0.607 | 0.74 |
| Ob2 | 0.722 | 0.964 | 0.666 | 0.634 | |
| CT‐based | 0.803 | 0.965 | 0.384 | 0.667 | |
| 0.727 | 0.959 | 0.884 | 0.707 | ||
| Inter‐observer reliability (ICC) | |||||
| Cup | Femoral stem | Neck length | Calcar | ||
| Acetate | Ob1 | 0.715 | 0.945 | 0.45 | 0.644 |
| CT‐based | Ob2 | 0.79 | 0.966 | 0.543 | 0.687 |
Time Comparison Between Acetate Templating and CT‐Based Planning
In the CT‐based preoperative planning group, the average planning time was 6.48 ± 1.55 min. In the acetate templating group, the average planning time was 6.12 ± 1.40 min (p = 0.015).
Intraoperative Results
Intraoperative measurements of neck length and neck cut length were made after the prosthesis was implanted, recorded as the actual neck length and neck cut length. The planned neck and neck cut lengths were measured based on the surgical plans. The absolute difference between the actual and planned measurements served as an indicator of the planning accuracy. The mean absolute difference for neck length was 4.275 ± 3.379 mm for acetate templating and 3.740 ± 4.547 mm for CT‐based planning (p = 0.122). The mean absolute difference in neck cut length was 3.052 ± 2.334 mm for acetate templating and 2.934 ± 2.685 mm for CT‐based planning (p = 0.731). There were no statistically significant differences in the mean absolute difference in neck length or neck cut length between acetate templating and CT‐based planning. A second femoral neck resection was required in 10 cases to adjust leg length and offset with the use of collared stems. The position of the implanted acetabular component was adjusted in four cases to allow for a better range of motion. Intraoperative complications, including periprosthetic fractures, have not been reported.
Discussion
Accuracy of CT‐Based Planning
In this study, CT‐based planning showed an accuracy of 93% for the acetabular component and 92% for the femoral component. Our results for CT‐based planning are consistent with the findings of other studies; Hassani et al. 13 reported 100% accuracy for the femoral component and 88% accuracy for the acetabular component with CT‐based three‐dimensional planning. Wu et al. 21 assessed CT‐based three‐dimensional planning in patients with a dysplastic acetabulum and reported a cup size accuracy of 100%. Ogawa et al. 22 reported 94% accuracy for cup size and 85% for stem size. Kobayashi et al. 23 used the multiplanar reformation technique to establish adjusted and calibrated CT images and printed them for templating. A total of 184 patients were included in the study. Their technique yielded 94.5% accuracy in predicting stem size and 95.5% accuracy in predicting cup size. The results of previous studies and those of our study suggest a high level of accuracy in CT‐based preoperative planning. This indicates consistency in the accuracy of the CT‐based planning technique in the literature, regardless of differences in software, patient selection, or observer. Senior surgeons and orthopaedic residents were selected as observers in different studies. In our study, residents conducted all preoperative planning and yielded equivalent accuracy compared with studies in which planning was conducted by senior surgeons 13 . The detailed and comprehensive assessment provided by the three‐dimensional planning system may compensate for the lack of experience in observers. Therefore, CT‐based planning may help surgeons with less experience to conduct preoperative planning as accurately as experienced surgeons.
Accuracy of Acetate Templating
Acetate templating accurately predicted acetabular component in 78.6% of cases and accurately predicted femoral component in 75% of cases. The reported accuracy of acetate templating differs widely in the literature. Dall et al. 9 and Eggli et al. 24 reported templating accuracies of over 90%. Petretta et al. 6 reported that acetate templating accurately predicted 77% of cup size and 75% of stem size. The average accuracy of two‐dimensional acetate templating was approximately 80% for the stem size and 77% for the cup size 2 . The quality of X‐ray radiographs has a significant influence on the accuracy of X‐ray‐based planning 3 . Variations in the projection angle and magnification rate in the X‐ray examination directly influence the sizing of the component. Studies that applied standardized techniques in X‐ray examination reported 70%–90% accuracy in predicting component size 6 , 24 . Unstandardized radiographic examination techniques may fail to provide a correct magnification rate and anatomical morphology, which leads to inaccurate planning. In the present study, the radiology department in our institution conducted all radiographs based on the same standardized technique. Standardized X‐ray examination techniques are the basis for accurate acetate templating.
Comparison Between the Two Techniques
In our series, the accuracy of acetate templating is inferior to that of CT‐based planning. CT‐based planning is three‐dimensional in nature. The planned implant position can be visualized from the sagittal, coronal, and axial views. In CT‐based planning, the femoral entry point was visualized in the sagittal and coronal plane, which may be helpful in avoiding the selection of smaller‐sized stems because of impingement of the anterior femoral cortex or varus/valgus placement of the stem. On the acetabular side, the anterior and posterior walls of the acetabular were well‐visualized, and cup coverage could be calculated in real‐time according to cup size, position, inclination, and anteversion. Compared with three‐dimensional planning, the information offered by two‐dimensional planning is limited. An example of a comparison between the two planning techniques is shown in Figure 5.
Fig. 5.
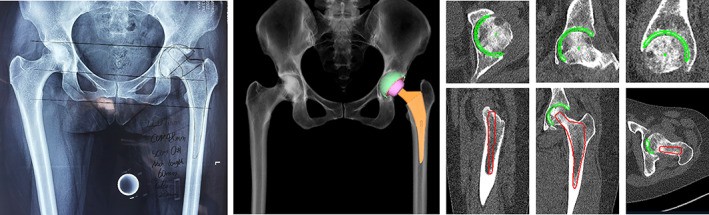
Example of comparison between the two planning methods. (A) Two‐dimensional acetate templating. (B) Three‐dimensional CT‐based planning.
Neck length and neck cut length were routinely measured during surgery. The predicted neck and neck cut lengths were more accurate in the CT‐based planning group. This might be explained by the fact that the lesser trochanter is more clearly visualized on reconstructed CT; therefore, the preoperative measurement on CT was closer to the intraoperative findings.
The fundamental difference between X‐ray acetate templating and CT planning is that CT planning provides information on a three‐dimensional scale. In acetate templating, preoperative planning is conducted only in the coronal plane. However, a plan that appears appropriate in the coronal plane may not be appropriate in the sagittal plane. For example, we have encountered cases in which the cup size determined by acetate templating is appropriate in the coronal plane; however, it did not match the anterior and posterior edges of the acetabulum as shown in the sagittal plane. In CT‐based planning, the component was placed considering both the coronal and sagittal planes. Other functions of CT‐based planning systems, including real‐time simulation and adjustment, have made it more convenient to modify surgical plans. One study reported approximately 40% accuracy for X‐ray planning and 70% accuracy for CT‐based planning 19 . The difference in accuracy between X‐ray and CT planning was greater in their study than in ours. This might be because complicated cases, such as DDH with Crowe II–IV classification, were not included in our study. The three‐dimensional assessment provided by CT‐based planning allowed for a better understanding of patients' anatomy and facilitated more realistic surgical simulation, which led to increased planning accuracy. We also hypothesize that the potential advantages of CT‐based planning may be more prominent in complicated cases.
Reproducibility and Influencing Factors of the Two Techniques
Inter‐observer and intra‐observer reliabilities were assessed using the ICC. Satisfactory agreement was reached in predicting the component size, suggesting good reproducibility for both planning methods. Previous studies have found a higher accuracy of templating in surgeons with higher experience level 3 , 25 . Observer experience and other covariates that may influence the accuracy of templating were also analyzed with generalized estimating equations, which showed that observer experience, surgical approach, diagnosis, and patient BMI were not factors with significant influence on planning reliability in either planning method.
Study Limitations
One limitation of this study is that most of the cases were AVN, which might not be the case in other institutions. In addition, both acetate templating and CT‐based planning were compared with the actual implanted components and intraoperative measurements. This assumes that the implanted component is of optimal size and that the intraoperative measurement is accurate, which might not be true in some cases. Another limitation relates to the statistical power. Although the current sample size provided sufficient power for statistical analysis, the inclusion of more patients would result in a statistical outcome with increased power.
Conclusion
CT‐based preoperative planning is more accurate in predicting the implant size for total hip arthroplasty than acetate templating. Observer experience, BMI, and surgical approach do not significantly influence the reliability of either planning method.
Conflicts of interests
All authors declare no conflict of interests.
Acknowledgement
This work was supported by the Non‐profit Central Research Institute Fund of Chinese Academy of Medical Science (Grant number A2020418300).
The analysis was mainly conducted in Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Science.
Contributor Information
Guixing Qiu, Email: qiugx_pumch@126.com.
Wenwei Qian, Email: qianww007@163.com.
References
- 1. Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, et al. Prevalence of Total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mainard D, Barbier O, Knafo Y, Belleville R, Mainard‐Simard L, Gross JB. Accuracy and reproducibility of preoperative three‐dimensional planning for total hip arthroplasty using biplanar low‐dose radiographs: a pilot study. Orthop Traumatol Surg Res. 2017;103:531–6. [DOI] [PubMed] [Google Scholar]
- 3. Gamble P, de Beer J, Petruccelli D, Winemaker M. The accuracy of digital templating in uncemented total hip arthroplasty. J Arthroplasty. 2010;25:529–32. [DOI] [PubMed] [Google Scholar]
- 4. Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. 2005;13:455–62. [DOI] [PubMed] [Google Scholar]
- 5. Bono JV. Digital templating in total hip arthroplasty. J Bone Joint Surg Am. 2004;2:118–22. [DOI] [PubMed] [Google Scholar]
- 6. Petretta R, Strelzow J, Ohly NE, Misur P, Masri BA. Acetate templating on digital images is more accurate than computer‐based templating for total hip arthroplasty. Clin Orthop Relat Res. 2015;473:3752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shaarani SR, McHugh G, Collins DA. Accuracy of digital preoperative templating in 100 consecutive uncemented total hip arthroplasties: a single surgeon series. J Arthroplasty. 2013;28:331–7. [DOI] [PubMed] [Google Scholar]
- 8. Colombi A, Schena D, Castelli CC. Total hip arthroplasty planning. EFORT Open Rev. 2019;4:626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dall DM, Miles AW, Juby G. Accelerated polymerization of acrylic bone cement using preheated implants. Clin Orthop Relat Res. 1986;211:148–50. [PubMed] [Google Scholar]
- 10. Efe T, El Zayat BF, Heyse TJ, Timmesfeld N, Fuchs‐Winkelmann S, Schmitt J. Precision of preoperative digital templating in total hip arthroplasty. Acta Orthop Belg. 2011;77:616–21. [PubMed] [Google Scholar]
- 11. Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad™ system. Arch Orthop Trauma Surg. 2010;130:1429–32. [DOI] [PubMed] [Google Scholar]
- 12. Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop Relat Res. 2008;466:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hassani H, Cherix S, Ek ET, Rüdiger HA. Comparisons of preoperative three‐dimensional planning and surgical reconstruction in primary cementless total hip arthroplasty. J Arthroplasty. 2014;29:1273–7. [DOI] [PubMed] [Google Scholar]
- 14. Tostain O, Debuyzer E, Benad K, Putman S, Pierache A, Girard J, et al. Ten‐year outcomes of cementless anatomical femoral implants after 3D computed tomography planning. Follow‐up note. Orthop Traumatol Surg Res. 2019;105:937–42. [DOI] [PubMed] [Google Scholar]
- 15. Osmani FA, Thakkar S, Ramme A, Elbuluk A, Wojack P, Vigdorchik JM. Variance in predicted cup size by 2‐dimensional vs 3‐dimensional computerized tomography‐based templating in primary total hip arthroplasty. Arthroplast Today. 2017;3:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asnis SE, Heller YY. Total hip arthroplasty Templating: a simple method to correct for radiograph magnification. Orthopedics. 2019;42:e322–5. [DOI] [PubMed] [Google Scholar]
- 17. Waldstein W, Bouché PA, Pottmann C, Faschingbauer M, Aldinger PR, Windhager R, et al. Quantitative and individualized assessment of the learning curve in preoperative planning of the acetabular cup size in primary total hip arthroplasty. Arch Orthop Trauma Surg. 2021;141:1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wako Y, Nakamura J, Miura M, Kawarai Y, Sugano M, Nawata K. Interobserver and Intraobserver reliability of three‐dimensional preoperative planning software in Total hip arthroplasty. J Arthroplasty. 2018;33:601–7. [DOI] [PubMed] [Google Scholar]
- 19. Huo J, Huang G, Han D, Wang X, Bu Y, Chen Y, et al. Value of 3D preoperative planning for primary total hip arthroplasty based on artificial intelligence technology. J Orthop Surg Res. 2021;16:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montgomery AA, Graham A, Evans PH, Fahey T. Inter‐rater agreement in the scoring of abstracts submitted to a primary care research conference. BMC Health Serv Res. 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu P, Liu Q, Fu M, Zhang Z, He S, Liao W, et al. Value of computed tomography‐based three‐dimensional pre‐operative planning in cup placement in Total hip arthroplasty with dysplastic acetabulum. J Invest Surg. 2019;32:607–13. [DOI] [PubMed] [Google Scholar]
- 22. Ogawa T, Takao M, Sakai T, Sugano N. Factors related to disagreement in implant size between preoperative CT‐based planning and the actual implants used intraoperatively for total hip arthroplasty. Int J Comput Assist Radiol Surg. 2018;13:551–62. [DOI] [PubMed] [Google Scholar]
- 23. Kobayashi H, Cech A, Kase M, Pagenstart G, Carrillon Y, O'Loughlin PF, et al. Pre‐operative templating in THA. Part II: a CT‐based strategy to correct architectural hip deformities. Arch Orthop Trauma Surg. 2020;140:551–62. [DOI] [PubMed] [Google Scholar]
- 24. Eggli S, Pisan M, Müller ME. The value of preoperative planning for total hip arthroplasty. J Bone Joint Surg Br. 1998;80:382–90. [DOI] [PubMed] [Google Scholar]
- 25. Carter LW, Stovall DO, Young TR. Determination of accuracy of preoperative templating of noncemented femoral prostheses. J Arthroplasty. 1995;10:507–13. [DOI] [PubMed] [Google Scholar]


