Abstract
Background
Rapid deployment of COVID-19 vaccines is challenging for safety surveillance, especially on adverse events of special interest (AESIs) that were not identified during the pre-licensure studies. This study evaluated the risk of hospitalisations for predefined diagnoses among the vaccinated population in Malaysia.
Methods
Hospital admissions for selected diagnoses between 1 February 2021 and 30 September 2021 were linked to the national COVID-19 immunisation register. We conducted self-controlled case-series study by identifying individuals who received COVID-19 vaccine and diagnosis of thrombocytopenia, venous thromboembolism, myocardial infarction, myocarditis/pericarditis, arrhythmia, stroke, Bell’s Palsy, and convulsion/seizure. The incidence of events was assessed in risk period of 21 days postvaccination relative to the control period. We used conditional Poisson regression to calculate the incidence rate ratio (IRR) and 95% confidence interval (CI) with adjustment for calendar period.
Results
There was no increase in the risk for myocarditis/pericarditis, Bell’s Palsy, stroke, and myocardial infarction in the 21 days following either dose of BNT162b2, CoronaVac, and ChAdOx1 vaccines. A small increased risk of venous thromboembolism (IRR 1.24; 95% CI 1.02, 1.49), arrhythmia (IRR 1.16, 95% CI 1.07, 1.26), and convulsion/seizure (IRR 1.26; 95% CI 1.07, 1.48) was observed among BNT162b2 recipients. No association between CoronaVac vaccine was found with all events except arrhythmia (IRR 1.15; 95% CI 1.01, 1.30). ChAdOx1 vaccine was associated with an increased risk of thrombocytopenia (IRR 2.67; 95% CI 1.21, 5.89) and venous thromboembolism (IRR 2.22; 95% CI 1.17, 4.21).
Conclusion
This study shows acceptable safety profiles of COVID-19 vaccines among recipients of BNT162b2, CoronaVac, and ChAdOx1 vaccines. This information can be used together with effectiveness data for risk-benefit analysis of the vaccination program. Further surveillance with more data is required to assess AESIs following COVID-19 vaccination in short- and long-term.
Keywords: COVID-19 vaccines, Adverse events of special interest, Safety, Self-controlled case series
1. Introduction
The administration of COVID-19 vaccines is viewed as the most promising approach to curb the pandemic. By the end of 2021, nearly nine billion doses of COVID-19 vaccines had been administered worldwide [1]. The vaccines have been proven to be safe and effective in clinical trials. Yet, as with the initiation of any new drugs, information on long-term safety and effectiveness is still being gathered during the post-marketing phase [2]. Rapid deployment of COVID-19 vaccines on a mass scale poses challenges for monitoring vaccine safety, including those not identified during the pre-licensure studies. Adverse effects following immunisation (AEFI) signal detection have primarily relied on passive surveillance reporting; however, it is often limited by incomplete information or under-reporting [3], [4]. As such, enhancement of the type and scope of vaccine monitoring activities, including the conduct of active surveillance activities and well-designed observational study could refine the collecting and processing of information on COVID-19 vaccine safety [5].
Variations in the safety profile between different vaccine platform are to be expected as the different mechanism was used to trigger an immune response. mRNA vaccines (BNT162b2 and mRNA-1273) and adenoviral vector vaccines (ChAdOx1, Ad26.COV2.S) have been most extensively studied due to their high prevalence in western countries. Little information is currently available on the inactivated vaccine (CoronaVac, BBIBP-CorV) that has significant uptake globally [6]. Since the initiation of the COVID-19 immunisation program in Malaysia in late February 2021, 65% of the adult population has been vaccinated by 31 August 2021 [7]. Diverse vaccine portfolios were administered to the population where the majority were inoculated with BNT162b2, CoronaVac, or ChAdOx1 vaccines while a smaller proportion received other vaccines including CanSino, Sinopharm BBIBP-CorV, Ad26.COV.S. With the different types of vaccines administered, questions are arising on the comparability of these vaccines, adverse events, and the required period of monitoring.
We established a case-based surveillance approach for adverse events of special interest (AESI) following COVID-19 vaccination using routinely collected administrative databases and health records. This project was initiated to improve the capacity of COVID-19 vaccine safety monitoring in the country and complement the current adverse events monitoring through the national passive surveillance system [8]. We evaluated the risk of serious adverse events potentially associated with COVID-19 vaccines by focusing on cases that require hospitalisation among the vaccinated population in Malaysia. In this paper, we present the interim analysis of the ongoing study that covers the first half period of the COVID-19 immunisation roll-out in Malaysia that evaluates the occurrence of AESIs among the vaccinated population. We compared the rate of pre-specified AESI between different vaccine platforms and evaluated whether there was an increased risk of events after COVID-19 vaccination.
2. Materials and methods
2.1. Data sources
Data on vaccination was retrieved from the Malaysia Vaccine Administration System (MyVAS) database that was launched to manage vaccination records for the National COVID-19 Immunisation programme in Malaysia (Supplement 1). The database includes information on vaccine brand, dates, and doses as well as demographic details and self-reported comorbidity for the vaccinated population in Malaysia.
Hospital admission data were obtained from the Malaysian Data Warehouse (MyHDW), a national health data repository that collects data from public and private hospitals in Malaysia. This data included diagnoses coded according to the International Classification of Disease (ICD-10), admission and discharge dates, and discharge status which was monitored and validated by the Health Informatics Centre, Ministry of Health Malaysia [9]. Between February and September 2021, data from 216 public and private hospitals were available for analysis. Outcome data were also obtained from two other sources: sentinel surveillance sites and the national pharmacovigilance database. This allowed for a more immediate identification of eligible cases to account for the lag in data accrual due to delays in submission to the central repository by the data providers. Eight public tertiary hospitals across Malaysia were selected as sentinel surveillance sites for this study. Eligible cases were sourced directly from locally held records at these hospitals i.e., hospital discharge database and cases were identified from ICD to 10 coded diagnoses. The pharmacovigilance database was used to identify outcome events from spontaneous AEFI reports submitted by healthcare professionals, pharmaceutical companies, or consumers. Only cases that require hospitalisation were included for analysis. Outcome data from all sources were cross-linked to check for overlapping records.
Data on COVID-19 confirmed cases were retrieved from the national COVID-19 surveillance system. This dataset was used to determine COVID-19 diagnosis status within the study population based on the infection date.
All datasets were linked using unique resident identification numbers to establish the cohort for this study. Record linkage was conducted using deterministic matching of identifiers and de-identified data was used for analysis.
2.2. Study design
We used self-controlled case-series (SCCS) study design to examine the associations between COVID-19 vaccination and outcome events by comparing the incidence of events across risk periods relative to the control period. SCCS design employs within-person comparison and time-invariant confounders (e.g., sex, ethnicity, lifestyle, chronic diseases) are self-adjusted [10].
2.3. Study population
The study population comprised individuals who received at least one dose of the COVID-19 vaccine and were admitted to hospitals with the outcome of interest between 1 February to 30 September 2021. Only the first event within this period was included in the analysis. Those who had records of hospital admissions for the same diagnosis in the two years before the study period were excluded. We also excluded individuals who were COVID-19 positive (i) during admission and (ii) in the 30-day interval before. To allow for sufficient follow-up time to capture the outcome, the cohort was restricted to vaccine doses administered up to 31 August 2021.
2.4. Outcome
Outcomes were hospital admission associated with the pre-selected diagnoses of interest: thrombocytopenia, venous thromboembolism, stroke, myocardial infarction, myocarditis/pericarditis, cardiac arrhythmia, Bell’s Palsy, and convulsion/seizures. Cases were identified using the ICD-10 diagnosis code in the diagnoses fields or cause of death recorded. The list of ICD-10 codes for each outcome is available in Supplement 2. The event date was the earliest date of hospital admission.
2.5. Exposure
Exposure was defined as receipt of one or more doses of COVID-19 vaccine. The date of vaccination was used as the date of exposure and individuals were classified according to the type of vaccine administered.
2.6. Statistical analysis
Characteristics of the study population (vaccinated individuals who developed the outcomes of interest) and the outcome events were described descriptively. The number of cases was tabulated by weeks since vaccination to describe event distribution. The absolute rate of events was calculated by dividing the total number of events by total doses administered and total persons vaccinated.
The SCCS method was used to investigate the association between outcome events and vaccination. Each patient follow-up time is divided into several periods: an exposed period (risk period) and an unexposed period (control period) (Supplement 3). The risk period comprised of 1 to 21 days after vaccination. The 21-day duration was defined based on literature and vaccine dose interval [11]. Given repeat exposures in which individuals may receive more than one dose of vaccine during the observation period, the risk period was defined as 21-day duration after any vaccine dose. The day of vaccination was defined as day 0 and included as a separate period. The 14 days before administration of the first vaccine dose was considered a pre-risk period since an event during this period is likely to affect the likelihood of receiving vaccination. All other observation times outside of these periods between 1 February 2021 and 30 September 2021 were defined as the control period.
The incidence rate ratio (IRR) of events in the risk period (exposed) and control period (unexposed) were calculated with the corresponding 95% confidence interval (CI). Each outcome was modelled separately using conditional Poisson regression with an offset of the length of risk periods. The models were adjusted for the month in the observation period to account for potential factors associated with calendar time. A 95% CI that did not include one indicates statistical significance. Using a risk period of 21 days, an observation period from 1 February to 30 September 2021, and IRR of 2.0 and 1.5, the sample size needed was 151 and 500, respectively, to estimate results with 80% power at 5% significant level. Reducing the IRR or risk period increases sample size requirements.
The rate ratio was also estimated for dose-effect where each dose of COVID-19 vaccine was regarded as a separate exposure and the risk periods were segmented by dose. Subgroup analysis was performed by age (<60 versus ≥ 60 years old) and sex of the patients to offset the risk of complications resulting from age and sex differences. Several measures were undertaken to assess assumptions of the SCCS method. To account for event dependent observation periods where the event may increase the risk of mortality and patient died before the end of observation, sensitivity analysis was carried out by excluding fatal events. Event dependent exposures were circumvented by including a pre-exposure risk period of 14 days. Another assumption of the SCCS method is that events must be independent of one another; therefore, analysis was restricted to only the first event.
Data were processed and analysed using STATA SE 15.0.
2.7. Ethical approval
This study was part of the project “Case-based monitoring of adverse events following COVID-19 vaccination – SAFECOVAC” that received approval from the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR-21-322-59745) which include a waiver of informed consent due to the use of secondary data for this research.
3. Results
By 31 August 2021, up to 35,201,509 doses of COVID-19 vaccines were administered to over 20 million individuals in Malaysia. BNT162b2 and CoronaVac vaccines accounted for 44% and 48%, respectively, 8% were ChAdOx1 while other vaccines accounted for less than 1%. Of individuals who had received at least one dose of the vaccine, 51% were aged 18–39 years and 16% were 60 years and older. Only 38% of individuals vaccinated with ChAdOx1 had completed two doses during this period, compared to over 70% for both BNT162b2 and CoronaVac recipients. The total number of doses administered and vaccinated persons by sex and age are shown in Supplement 4.
3.1. Event characteristics
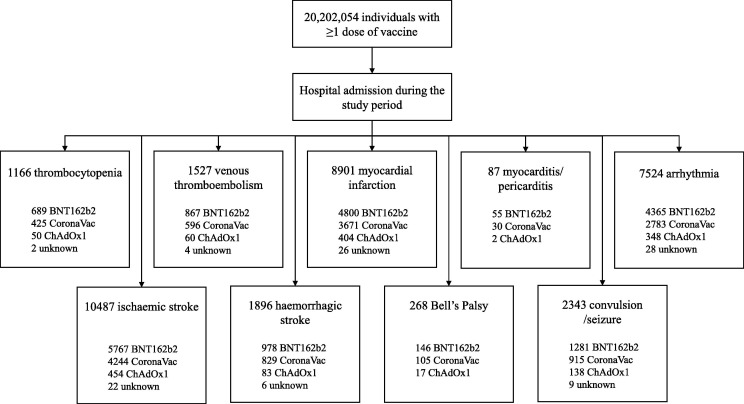
Fig. 1 shows an overview of patients included in the primary analysis by the individual outcome events for hospital admissions between 1 February 2021 and 30 September 2021 among the vaccinated population. The number of events ranged from 87 (myocarditis/pericarditis) to 10,487 (ischaemic stroke). We presented results for BNT162b2, CoronaVac, and ChAdOx1 vaccines as the events with other vaccine types were too small. Events that occurred in the first three weeks after vaccination was approximately 45% of all events observed in the postvaccination period (Supplement 5); the event distribution pattern was similarly observed with all three vaccines.
Fig. 1.
Flow diagram of study population. The study period is from 1 February 2021 to 30 September 2021. Unknown refers to those whose vaccine type was not available.
Table 1 shows the characteristics of events and demographics of patients who had hospital admissions for the outcome events in the 21 days following vaccination. More events were recorded after the first dose of vaccine than the second dose. Patients aged 60 years and older accounted for more than half of the cases, but the mean age was younger for those who had myocarditis/pericarditis (43.6 years), Bell’s Palsy (50.1 years), and convulsion/seizures (50.6 years). There was a preponderance of males among those with myocardial infarction (75%), stroke (60%), and myocarditis/pericarditis (60%). Nearly 50% of the patients who had myocardial infarction, arrhythmia, and stroke were reported to have hypertension.
Table 1.
Characteristics of events that occurred in 21 days after either dose of COVID-19 vaccine among vaccinated population in Malaysia from 1 February 2021 to 30 September 2021.
|
Thrombo cytopenia |
Venous thrombo embolism |
Myocardial infarction | Myocarditis / pericarditis | Arrhythmia | Stroke, ischaemic | Stroke haemorrhagic | Bell’s Palsy | Convulsion / seizure | |
|---|---|---|---|---|---|---|---|---|---|
| Total event | 206 | 307 | 1495 | 25 | 1375 | 1840 | 401 | 53 | 401 |
| Dose 1 | 104 (50.5) | 187 (60.9) | 799 (53.4) | 18 (72.0) | 758 (55.1) | 1005 (54.6) | 236 (58.9) | 33 (62.3) | 218 (54.4) |
| Dose 2 | 102 (49.5) | 120 (39.1) | 696 (46.6) | 7 (28.0) | 617 (44.9) | 835 (45.4) | 165 (41.1) | 20 (37.7) | 183 (45.6) |
| Died | – | – | 3 (0.2) | – | – | 3 (0.2) | 2 (0.5) | – | – |
| Vaccine platform | |||||||||
| BNT162b2 | 130 (63.1) | 166 (54.1) | 796 (53.2) | 14 (56.0) | 834 (60.7) | 1006 (54.7) | 199 (49.6) | 27 (50.9) | 227 (56.6) |
| CoronaVac | 64 (31.1) | 122 (39.7) | 644 (43.1) | 9 (36.0) | 485 (35.3) | 768 (41.7) | 186 (46.4) | 21 (39.6) | 153 (38.2) |
| ChAdOx1 | 12 (5.8) | 18 (5.9) | 55 (3.7) | 2 (8.0) | 53 (3.9) | 64 (3.5) | 14 (3.5) | 5 (9.4) | 21 (5.2) |
| Others | – | 1 (0.3) | – | – | 3 (0.2) | 2 (0.1) | 2 (0.5) | – | – |
| Male | 116 (56.3) | 170 (55.4) | 1121 (75.0) | 15 (60.0) | 731 (52.8) | 1096 (59.6) | 247 (61.6) | 27 (50.9) | 229 (57.1) |
| Age (years) | |||||||||
| Mean (SD) | 57.5 (16.7) | 56.6 (15.2) | 60.3 (12.7) | 43.6 (15.2) | 62.8 (16.6) | 63.6 (12.6) | 59.9 (16.4) | 50.1 (15.2) | 50.6 (18.4) |
| 18–39 | 40 (19.4) | 52 (16.9) | 97 (6.5) | 11 (44.0) | 176 (12.7) | 75 (4.1) | 48 (12.0) | 15 (28.3) | 142 (35.4) |
| 40–59 | 63 (30.6) | 110 (35.8) | 589 (39.4) | 10 (40.0) | 287 (20.7) | 577 (31.4) | 130 (32.4) | 20 (37.7) | 115 (28.7) |
| 60+ | 103 (50.0) | 145 (47.2) | 809 (54.1) | 4 (16.0) | 921 (66.5) | 1188 (64.6) | 222 (55.4) | 18 (34.0) | 144 (35.9) |
| Ethnicity | |||||||||
| Malay | 128 (62.1) | 208 (67.8) | 887 (59.3) | 11 (44.0) | 814 (58.8) | 1070 (58.2) | 228 (56.9) | 29 (54.7) | 245 (61.1) |
| Chinese | 33 (16.0) | 40 (13.0) | 258 (17.3) | 5 (20.0) | 350 (25.3) | 452 (24.6) | 100 (24.9) | 16 (30.2) | 70 (17.5) |
| Indian | 16 (7.8) | 20 (6.5) | 186 (12.4) | 4 (16.0) | 69 (5.0) | 140 (7.6) | 16 (4.0) | 5 (9.4) | 40 (10.0) |
| Others | 29 (14.1) | 35 (11.4) | 137 (9.2) | 5 (20.0) | 143 (10.3) | 157 (8.5) | 50 (12.5) | 2 (3.8) | 41 (10.2) |
| Non-Malaysian | – | 3 (1.0) | 27 (1.8) | – | 7 (0.5) | 18 (1.0) | 7 (1.7) | 1 (1.9) | 4 (1.0) |
| Comorbidities | |||||||||
| Hypertension | 90 (43.7) | 129 (42.0) | 759 (50.8) | 4 (16.0) | 735 (53.1) | 1025 (55.7) | 182 (45.4) | 18 (34.0) | 119 (29.7) |
| Diabetes | 56 (27.2) | 91 (29.6) | 556 (37.2) | 4 (16.0) | 426 (30.8) | 721 (39.2) | 94 (23.4) | 15 (28.3) | 73 (18.2) |
| Heart disease | 17 (8.3) | 19 (6.2) | 268 (17.9) | 3 (12.0) | 293 (21.2) | 186 (10.1) | 30 (7.5) | 5 (9.4) | 23 (5.7) |
| Dyslipidaemia | – | – | – | – | – | – | 1 (0.2) | – | 1 (0.2) |
Data are presented as n (%) except otherwise stated. Abbreviation: SD, standard deviation. Numbers may not add up to the total due to the missing value.
3.2. Absolute rate of events
Table 2 shows the absolute rate for each event by vaccine type. Ischaemic stroke was the most frequent and the absolute event rate within 21 days of any vaccination was between 33 and 115 cases per million doses administered. This was followed by myocardial infarction (28 to 90 cases per million doses) and arrhythmia (27 to 95 cases per million doses). The event rate for others was much lower at less than 30 cases per million doses for each diagnosis. The absolute event rates appeared to be slightly higher among BNT162b2 vaccine recipients compared to CoronaVac and ChAdOx1 vaccine recipients for most events.
Table 2.
Absolute rate of outcome events within 21 days of COVID-19 vaccination by vaccine type.
| BNT162b2 | CoronaVac | ChAdOx1 | |
|---|---|---|---|
| Thrombocytopenia | |||
| No. of events | 130 | 64 | 12 |
| Event rate per 1 million doses administered | 8.45 | 3.76 | 4.37 |
| Event rate per 1 million vaccinated persons | 14.88 | 6.83 | 6.10 |
| Venous thromboembolism | |||
| No. of events | 166 | 122 | 18 |
| Event rate per 1 million doses administered | 10.79 | 7.16 | 6.56 |
| Event rate per 1 million vaccinated persons | 19.00 | 13.02 | 9.14 |
| Myocardial infarction | |||
| No. of events | 796 | 644 | 55 |
| Event rate per 1 million doses administered | 51.73 | 37.82 | 20.04 |
| Event rate per 1 million vaccinated persons | 91.12 | 68.73 | 27.94 |
| Myocarditis/pericarditis | |||
| No. of events | 14 | 9 | 2 |
| Event rate per 1 million doses administered | 0.91 | 0.53 | 0.73 |
| Event rate per 1 million vaccinated persons | 1.60 | 0.96 | 1.02 |
| Arrhythmia | |||
| No. of events | 834 | 485 | 53 |
| Event rate per 1 million doses administered | 54.20 | 28.48 | 19.31 |
| Event rate per 1 million vaccinated persons | 95.47 | 51.76 | 26.92 |
| Stroke, ischaemic | |||
| No. of events | 1006 | 768 | 64 |
| Event rate per 1 million doses administered | 65.38 | 45.10 | 23.32 |
| Event rate per 1 million vaccinated persons | 115.16 | 81.96 | 32.51 |
| Stroke, haemorrhagic | |||
| No. of events | 199 | 186 | 14 |
| Event rate per 1 million doses administered | 12.93 | 10.92 | 5.10 |
| Event rate per 1 million vaccinated persons | 22.78 | 19.85 | 7.11 |
| Bell's Palsy | |||
| No. of events | 27 | 21 | 5 |
| Event rate per 1 million doses administered | 1.75 | 1.23 | 1.82 |
| Event rate per 1 million vaccinated persons | 3.09 | 2.24 | 2.54 |
| Convulsion/seizure | |||
| No. of events | 227 | 153 | 21 |
| Event rate per 1 million doses administered | 14.75 | 8.98 | 7.65 |
| Event rate per 1 million vaccinated persons | 25.99 | 16.33 | 10.67 |
Denominator is total vaccine doses administered and total individuals vaccinated up to 31 August 2021.
3.3. Incidence rate ratio of events
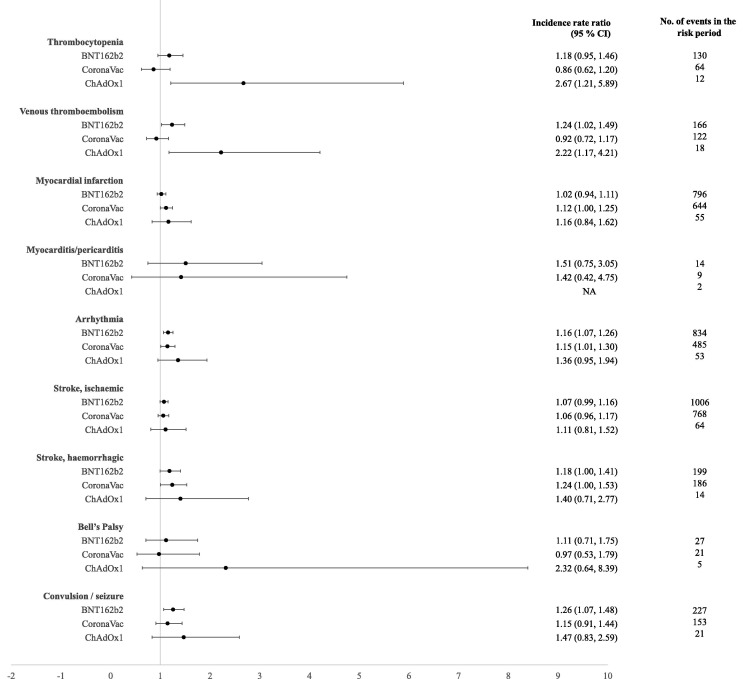
Fig. 2 shows the incidence rate ratio of events in the 21-day risk period following either dose of BNT162b2, CoronaVac, and ChAdOx1 vaccines compared to the control period. IRR estimates accounting for the vaccine dose effect are summarised in Table 3 and the IRR in all subintervals within the risk and control periods are shown in Supplement 6.
Fig. 2.
Incidence rate ratios and 95% confidence intervals of outcome events in the 21-day risk period after either dose of BNT162b2, CoronaVac, and ChAdOx1 vaccines compared with outcome events in the control period, adjusted for calendar month between 1 February 2021 to 30 September 2021. Abbreviation: NA, not available.
Table 3.
Incidence rate ratios of outcome events in the 21-day risk period after COVID-19 vaccination by vaccine type and dose number, adjusted for calendar month between 1 February 2021 to 30 September 2021.
|
Dose 1 |
Dose 2 |
|||||
|---|---|---|---|---|---|---|
| No. of events |
Incidence rate ratio (95% CI) |
No. of events |
Incidence rate ratio (95% CI) |
|||
| Thrombocytopenia | ||||||
| BNT162b2 | 66 | 1.09 (0.83, 1.43) | 64 | 1.29 (0.98, 1.70) | ||
| CoronaVac | 28 | 0.68 (0.44, 1.05) | 36 | 1.09 (0.73, 1.62) | ||
| ChAdOx1 | 10 | 2.58 (1.13, 5.90) | 2 | 3.44 (0.64, 18.6) | ||
| Venous thromboembolism | ||||||
| BNT162b2 | 103 | 1.34 (1.07, 1.67) | 63 | 1.09 (0.83, 1.44) | ||
| CoronaVac | 68 | 0.94 (0.71, 1.26) | 54 | 0.89 (0.65, 1.22) | ||
| ChAdOx1 | 15 | 2.52 (1.30, 4.92) | 3 | 1.24 (0.33, 4.66) | ||
| Myocardial infarction | ||||||
| BNT162b2 | 409 | 0.97 (0.87, 1.08) | 387 | 1.08 (0.97, 1.21) | ||
| CoronaVac | 356 | 1.16 (1.02, 1.32) | 288 | 1.06 (0.92, 1.23) | ||
| ChAdOx1 | 34 | 1.02 (0.69, 1.51) | 21 | 1.58 (0.93, 2.67) | ||
| Myocarditis/pericarditis | ||||||
| BNT162b2 | 9 | 1.52 (0.67, 3.46) | 5 | 1.13 (0.41, 3.09) | ||
| CoronaVac | 7 | 1.97 (0.57, 6.74) | 2 | 0.67 (0.12, 3.83) | ||
| ChAdOx1 | 2 | ** | – | – | ||
| Arrhythmia | ||||||
| BNT162b2 | 437 | 1.12 (1.01, 1.25) | 397 | 1.19 (1.07, 1.33) | ||
| CoronaVac | 276 | 1.18 (1.02, 1.37) | 209 | 1.10 (0.93, 1.29) | ||
| ChAdOx1 | 42 | 1.51 (1.03, 2.23) | 11 | 0.96 (0.49, 1.90) | ||
| Stroke, ischaemic | ||||||
| BNT162b2 | 535 | 1.05 (0.95, 1.15) | 471 | 1.11 (1.00, 1.23) | ||
| CoronaVac | 421 | 1.05 (0.93, 1.18) | 347 | 1.07 (0.94, 1.22) | ||
| ChAdOx1 | 47 | 1.14 (0.80, 1.63) | 17 | 1.01 (0.58, 1.76) | ||
| Stroke, haemorrhagic | ||||||
| BNT162b2 | 119 | 1.29 (1.05, 1.59) | 80 | 1.05 (0.82, 1.34) | ||
| CoronaVac | 105 | 1.31 (1.02, 1.68) | 81 | 1.16 (0.89, 1.52) | ||
| ChAdOx1 | 11 | 1.32 (0.63, 2.79) | 3 | 1.80 (0.47, 6.94) | ||
| Bell’s Palsy | ||||||
| BNT162b2 | 17 | 1.32 (0.77, 2.24) | 10 | 0.88 (0.45, 1.73) | ||
| CoronaVac | 12 | 1.06 (0.52, 2.18) | 9 | 0.87 (0.40, 1.92) | ||
| ChAdOx1 | 4 | 2.88 (0.74, 11.2) | 1 | 1.09 (0.10, 12.4) | ||
| Convulsion/seizure | ||||||
| BNT162b2 | 116 | 1.15 (0.94, 1.42) | 111 | 1.39 (1.12, 1.72) | ||
| CoronaVac | 83 | 1.12 (0.86, 1.47) | 70 | 1.17 (0.87, 1.57) | ||
| ChAdOx1 | 19 | 1.55 (0.88, 2.75) | 2 | 0.85 (0.18, 3.94) | ||
** Values are suppressed for total event <5. Rate ratio estimates for comparison with control period of day 22 after the last vaccine dose until 30 September 2021 (post vaccination control) and from 1 February 2021 until 15 days before vaccination (pre-vaccination control). Day of vaccination (day 0) and 14 days before vaccination (pre-risk period) were included as separate risk periods. Abbreviations: CI, confidence interval; n, number of events in the risk period.
In the 21 days following either vaccine dose, no significant increased risk was found for myocarditis/pericarditis, Bell’s Palsy, stroke, and myocardial infarction in all vaccine platforms (Fig. 2). Among persons receiving BNT162b2 vaccine, the IRRs showed a slight elevation in the risk of venous thromboembolism, arrhythmia, and convulsion/seizure. In the analysis by vaccine dose, a significant association for venous thromboembolism was observed following the first dose (IRR 1.34; 95% CI 1.07, 1.67) whereas the risk of convulsion/seizure was significant after the second dose (IRR 1.39; 95% CI 1.12, 1.72) of BNT162b2 vaccine (Table 3). Overall estimates showed no association between CoronaVac vaccine and all outcomes, except arrhythmia. When the analysis was conducted by vaccine dose number, the risk appears to be slightly increased for myocardial infarction (IRR 1.16; 95% CI 1.02, 1.32), arrhythmia (IRR 1.18; 95% CI 1.02, 1.37), and haemorrhagic stroke (IRR 1.31; 95% CI 1.02, 1.68) after the first dose of CoronaVac vaccine. ChAdOx1 vaccine showed a significant association for several outcome events including thrombocytopenia and venous thromboembolism, but the number of events was smaller in this group resulting in a wider confidence interval.
3.4. Subgroup analyses by age group and sex and sensitivity analysis
The IRR estimates for subgroup analyses by age and sex are shown in Supplement 7. Stratification by sex showed the risk of venous thromboembolism and arrhythmia among BNT162b2 and CoronaVac recipients were higher in males. On the other hand, the risk of ischaemic stroke was significant among female CoronaVac recipients. Age effect was observed where patients aged ≥ 60 years have a higher risk of stroke, arrhythmia, and convulsion/seizure than their younger counterparts. No significant association was observed for any outcome among patients younger than 60 years old.
In a sensitivity analysis, cases of fatal admissions were excluded. Results of this additional analysis were consistent with those of the main findings which suggest a minimal bias for event dependent observation period (data not shown).
4. Discussion
This analysis of a population-based study covering over 20 million vaccinated individuals in Malaysia shows the risk of hospitalisation for predefined diagnoses among those vaccinated with BNT162b2, CoronaVac, and ChAdOx1 vaccines. We found that majority of events that occurred post-exposure were largely concentrated during the first three weeks after vaccination. We evaluated the relative risk of outcome events in 21 days following vaccination compared to the control period and found no significant increased risk for most events. Despite the statistically significant association for several events after vaccination, the point estimate of incidence rate ratios and lower limit of the 95% confidence interval were close to 1. The absolute number of events was low in all three vaccines.
We did not find a significant association between COVID-19 vaccination and cardiovascular complications, except arrhythmia. Cases of increased or irregular heart rate could be a physiological or stress-related response to vaccination [12], [13] which could explain the findings where most events occurred during 1–7 days after vaccination. Arrhythmia could also be triggered by a myocardial injury and is not an uncommon occurrence in myocarditis or pericarditis [14], [15]. Although no study has found an association between arrhythmia and COVID-19 vaccines, our findings suggest that arrhythmia following vaccination in our population is an AESI of concern. We also observed a small increased risk of venous thromboembolism after BNT162b2 and ChAdOx1 vaccination while the higher risk of thrombocytopenia was significant for ChAdOx1 vaccine. Although the elevated risk of these events indicates potential association with the vaccines, the low absolute number of events, especially within ChAdOx1 has to be noted and the significant association in the main and subgroup analyses comes with a broad confidence interval.
Our results are consistent with and extend the findings of prior studies on the risk of serious adverse events following COVID-19 vaccination [11], [16], [17]. Vaccine-induced immune thrombotic thrombocytopenia (VITT) is one of the rare, serious events reported after COVID-19 vaccination which was more common with ChAdOx1 than with other COVID-19 vaccines [18], [19]. A recent study from England based on clinically validated cases from hospitals estimated the risk of thrombosis with thrombocytopenia syndrome after ChAdOx1 vaccination was higher in 18 to 39 years old than in the older population, highlighting the difference in risk between different age groups [20]. In our study, the absolute event rate for thrombosis and thrombocytopenia cases was lower than those observed in other countries [16], [21], [22]. Increased risk of myocarditis following receipt of mRNA COVID-19 vaccines has been described in several studies [23], [24], [25]. We did not observe the elevated risk of myocarditis/pericarditis for the population in Malaysia vaccinated with BNT162b2 during the study period and the number of events was too small for meaningful comparison by subgroup. Information to date indicates that these events occur more commonly in male, adolescent/young adults following mRNA vaccination [26]. The present analysis covers the period when vaccination for adolescents in Malaysia was just initiated (September 2021) and during this early period, there was no record of hospitalisation for the outcome events among the adolescent group. In terms of neurological complications, there were mixed findings on the association with COVID-19 vaccines. Previous study from the UK observed an increased risk of Bell’s Palsy and Guillain-Barre syndrome following ChAdOx1 vaccination [27] while a study from Hong Kong reported risk of Bell’s Palsy linked to CoronaVac vaccine [28]. Li et al. conducted a population-based study in the United Kingdom and Spain [29] and did not find safety signal for the risk of neurological events among those vaccinated with BNT162b2 and ChAdOx1 vaccine. Our findings are in line with the latter study, with no elevated risk of Bell’s Palsy or Guillain-Barre syndrome seen for either BNT162b2, ChAdOx1, or CoronaVac. Compared to other vaccines, there was less information available on real-world data of AESIs among CoronaVac recipients. Our study further showed that the risk for most AESIs was found to be not significantly increased in those receiving CoronaVac. There was a slight elevation in the risk of myocardial infarction and ischaemic stroke after CoronaVac vaccination when the analysis was conducted by dose and sex-specific, which was not observed with other vaccines. Yet, this result must be interpreted cautiously since subgroup analyses lack power, and evidence on serious events with CoronaVac vaccination are still accruing for comparison on the magnitude of risk.
Although many cardiovascular and cerebrovascular events were reported following vaccination, a causality assessment needs to be conducted to confirm the associations because these events are prevalent among the population in Malaysia. Myocardial infarction and ischaemic stroke are the top leading cause of death in Malaysia for over a decade where on average, 90 to 100 hospital admissions occurred each day due to these events [30], [31]. Despite including incidence cases in the analysis, the study cohort still comprised patients with other risk factors and comorbidities which could lead to the development of events regardless of vaccination status. The risk of events can also be attributed to the population selected for vaccination. The elderly and high-risk populations were prioritised for vaccination and some events such as cardiovascular complications are more commonly seen in these populations. Due to the opt-in policy for ChAdOx1 vaccination in Malaysia, this group include those of younger age categories. This is unlike other countries that limit the use of ChAdOx1 in the older age group amid safety concerns that are more prevalent in the younger population [32]. Therefore, the population vaccinated with ChAdOx1 is considered to be “healthier” than those who received other vaccines since individuals without health concerns are more likely to sign up for this cohort. Moreover, there were substantially fewer second doses of ChAdOx1 vaccines given during the study period due to the longer dose interval of 9–12 weeks for the administration of the second dose according to the policy and recommendation adopted in Malaysia [33].
Based on the distribution of event occurrence over time, our finding suggests that the 3-week time period following vaccination is a crucial period for monitoring AESI and any event that occurs during this period warrants further investigation on the potential association to vaccines. Nevertheless, events presented later should not be disregarded since both short- and long-term risks need to be assessed. In studies evaluating the association between AESI and COVID-19 vaccines, the period considered at risk ranged between 21 and 28 days after vaccination with at least 3 months duration for the overall surveillance period [16], [17], [22], [34]. The length of time for monitoring AESI after vaccination should have a sufficient duration of follow-up to include events that occur at different time points relative to vaccine exposure to detect any important risk [5], [35].
4.1. Strength and limitations
To the best of our knowledge, this is one of the few large population-based studies that provide evidence of risk estimate for serious adverse events after vaccination in the population between the three different vaccine platforms. Our study addresses the limitation of data capture via a passive surveillance system by including a longer follow-up period to monitor outcome occurrence among vaccinated populations and identify the potential association with the vaccine. Furthermore, ascertainment of hospitalisations and vaccination status was conducted independently which allow us to capture more events and we utilised the SCCS design to provide estimates of risk on both relative scale (incidence rate ratios) and absolute scale (rate per million vaccinated persons). The SCCS design is an established method in vaccine safety study that uses within-person comparison to control all constant confounding factors during the study period. We also adjusted for the calendar period to account for potential temporal confounding. In this study, we provide additional context on a range of outcomes among CoronaVac vaccinees, which has not been frequently reported in large observational studies so far.
There are several limitations in this study that we acknowledge. We used secondary databases of the vaccinated and hospitalised; hence we cannot completely rule out misclassification bias and unmeasured confounding. We used hospitalisations as the study outcomes which captures events of a more serious nature that are admitted to hospitals for diagnosis or treatment. It is possible that some people who have had vaccination and developed the event were not hospitalised or died before being admitted. Ascertainment of outcomes was based on diagnosis codes, which might overestimate the risk without including other parameters to define the case; for instance, diagnosis of VITT and myocarditis are usually accompanied by laboratory parameters. Lastly, some of the predefined outcome events are rare, resulting in limited statistical power and wide confidence interval. Larger study cohorts and additional data over extended period of time will be useful for more detailed analysis.
4.2. Policy implications
Compared with the total number of vaccine doses given, our findings show that the incidence of all serious events are relatively low. There was an overall increased risk of certain events within the 21 days after vaccination compared to the control period, but the weak association requires further consideration of the clinical importance of the results. Given that COVID-19 remain prevalent and the vaccines provide nearly 90% protection against infection, the benefits of vaccination still far outweigh the risks [36]. Furthermore, studies have demonstrated that the risk of serious events associated with the COVID-19 infection itself was higher than the risk from vaccination [16], [23], [27]. Although the estimated risks of these serious adverse events in our population might be higher or lower than those reported in other countries, these initial findings provide valuable information that could help to inform clinical decision making and policymakers, especially in the risk-benefit assessments and subsequent immunisation plan.
5. Conclusion
Overall, the study demonstrates the safety of COVID-19 vaccines concerning the study outcomes among recipients of BNT162b2, CoronaVac, and ChAdOx1 vaccines. Our findings suggest that serious events of concern within our population include thrombocytopenia, venous thromboembolism, convulsion/seizure, and arrhythmia, although the numbers were relatively small and further exploration of the causal link with vaccination is warranted. More importantly, the overall benefits of COVID-19 vaccines outweigh the potential risks and this information needs to be interpreted in conjunction with vaccine effectiveness data for risk-benefit analysis of the vaccination program. Ongoing monitoring of COVID-19 vaccine safety is required and the information will be regularly updated as more data accumulate to assess short- and long-term complications with vaccination.
Funding
This research was supported by a grant from the Ministry of Health Malaysia – Sukuk Prihatin (NMRR-21-822-59745). The funding body had no role in the study design, data analysis, or the presentation of data and writing of the manuscript.
The SAFECOVAC study group
Members of the SAFECOVAC study group are listed in Supplement 8.
CRediT authorship contribution statement
Norazida Ab Rahman: Conceptualization, Methodology, Formal analysis, Writing – original draft, Visualization, Funding acquisition. Ming Tsuey Lim: Investigation, Writing – review & editing, Project administration. Fei Yee Lee: Investigation, Writing – review & editing, Project administration. Sing Chet Lee: Investigation, Writing – review & editing. Azuana Ramli: Investigation, Writing – review & editing. Siti Nurhafizah Saharudin: Investigation, Writing – review & editing. Teck Long King: Investigation, Writing – review & editing. Emelyne Bani Anak Jam: Investigation, Writing – review & editing. Nor Aliya Ayub: Investigation, Writing – review & editing. Raj Kumar Sevalingam: Investigation, Writing – review & editing. Rashidah Bahari: Investigation, Writing – review & editing. Nor Nadziroh Ibrahim: Investigation, Writing – review & editing. Fatihah Mahmud: Investigation, Writing – review & editing. Sheamini Sivasampu: . Kalaiarasu M Peariasamy: Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank the Director General of Health Malaysia for his permission to publish this study. We also thank all the investigators and collaborators for their immense contribution and support. We would like to acknowledge all data providers who make data available for research and research assistants who were involved in data collection.
Data Availability
The data that supports the findings of this study are available within the article and its supplementary materials. Access to datasets is provided by the corresponding data custodians for analysis for this study, but we have no permission to make generated datasets available. Malaysia COVID-19 vaccine administration data are available at https://github.com/MoH-Malaysia/covid19-public, redacted of personal identifying information.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.05.075.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, et al. Coronavirus (COVID-19) Vaccinations Published online at OurWorldInData.org; 2020 [Available from: https://ourworldindata.org/covid-vaccinations.
- 2.Chandler R.E. Optimizing safety surveillance for COVID-19 vaccines. Nat Rev Immunol. 2020;20(8):451–452. doi: 10.1038/s41577-020-0372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin M.R., Braun M.M., Bart K.J. What should an ideal vaccine postlicensure safety system be? Am J Public Health. 2009;99(S2):S345–S350. doi: 10.2105/AJPH.2008.143081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei J., Balakrishnan M.R., Gidudu J.F., Zuber P.L.F. Use of a new global indicator for vaccine safety surveillance and trends in adverse events following immunization reporting 2000–2015. Vaccine. 2018;36(12):1577–1582. doi: 10.1016/j.vaccine.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 vaccines: safety surveillance manual, second edition. Geneva: World Health Organization; 2021.
- 6.Mallapaty S. China's COVID vaccines have been crucial - now immunity is waning. Nature. 2021;598(7881):398–399. doi: 10.1038/d41586-021-02796-w. [DOI] [PubMed] [Google Scholar]
- 7.COVID-19 Immunisation Task Force Malaysia (CITF-Malaysia) Ministry of Health Malaysia. Open data on Malaysia's National COVID-19 Immunisation Programme. 2021 [Available from: https://github.com/citf-malaysia/citf-public.
- 8.Malaysian Pharmacovigilance Guidelines (Second Edition). Selangor, Malaysia: National Pharmaceutical Regulatory Agency (NPRA), Ministry of Health Malaysia; 2016.
- 9.Malaysian Health Data Warehouse (MyHDW) - 2015-2016 Start up: initiation. Malaysia: Health Informatics Centre, Planning Division, Ministry of Health Malaysia; 2017.
- 10.Weldeselassie Y.G., Whitaker H.J., Farrington C.P. Use of the self-controlled case-series method in vaccine safety studies: review and recommendations for best practice. Epidemiol Infect. 2011;139(12):1805–1817. doi: 10.1017/S0950268811001531. [DOI] [PubMed] [Google Scholar]
- 11.Jabagi M.J., Botton J., Bertrand M., Weill A., Farrington P., Zureik M., et al. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA. 2022;327(1):80. doi: 10.1001/jama.2021.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quer G., Gadaleta M., Radin J.M., Andersen K.G., Baca-Motes K., Ramos E., et al. medRxiv : the preprint server for health sciences. 2021. The Physiologic Response to COVID-19 Vaccination. [Google Scholar]
- 13.Taylor S., Asmundson G.J.G. Immunization stress-related responses: Implications for vaccination hesitancy and vaccination processes during the COVID-19 pandemic. J Anxiety Disord. 2021;84:102489. doi: 10.1016/j.janxdis.2021.102489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiravi A.A., Ardekani A., Sheikhbahaei E., Heshmat-Ghahdarijani K. Cardiovascular Complications of SARS-CoV-2 Vaccines: An Overview. Cardiol. Therapy. 2022;11(1):13–21. doi: 10.1007/s40119-021-00248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheuermeyer F.X., Yoo J., Greene M., O’Donnell S. Atrial fibrillation as a precursor of mRNA-1273 SARS-CoV-2 vaccine-induced pericarditis. Can J Emerg Med. 2022;24(2):230–232. doi: 10.1007/s43678-021-00250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ (Clinical research ed). 2021;374:n1931. [DOI] [PMC free article] [PubMed]
- 17.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA. 2021;326(14):1390. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavord S., Scully M., Hunt B.J., Lester W., Bagot C., Craven B., et al. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N Engl J Med. 2021;385(18):1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al‐Ali D., Elshafeey A., Mushannen M., Kawas H., Shafiq A., Mhaimeed N., et al. Cardiovascular and haematological events post COVID-19 vaccination: A systematic review. J Cell Mol Med. 2022;26(3):636–653. doi: 10.1111/jcmm.17137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins H., Andrews N., Stowe J., Amirthalingam G., Ramsay M., Bahra G., et al. Risk of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination prior to the recognition of vaccine-induced thrombocytopenia and thrombosis: A self-controlled case series study in England. Res Pract Thrombosis Haemostasis. 2022;6(3) doi: 10.1002/rth2.v6.310.1002/rth2.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson C.R., Shi T., Vasileiou E., Katikireddi S.V., Kerr S., Moore E., et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiteley W.N., Ip S., Cooper J.A., Bolton T., Keene S., Walker V., et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLoS Med. 2022;19(2):e1003926. doi: 10.1371/journal.pmed.1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., et al. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. New Engl. J Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozkurt B., Kamat I., Hotez P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention National Center for Immunization & Respiratory Diseases. COVID-19 vaccine safety update: Advisory Committee on Immunization Practices (ACIP) [Webinar] Atlanta, GA: Centers for Disease Control and Prevention; 2021 [presented 23 Jun 2021; cited 20 Dec 2021]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-COVIDShimabukuro-508.pdf.
- 27.Patone M., Handunnetthi L., Saatci D., Pan J., Katikireddi S.V., Razvi S., et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144–2153. doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan E.Y.F., Chui C.S.L., Lai F.T.T., Chan E.W.Y., Li X., Yan V.K.C., et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Raventós B, Roel E, Pistillo A, Martinez-Hernandez E, Delmestri A, et al. Association between covid-19 vaccination, SARS-CoV-2 infection, and risk of immune mediated neurological events: population based cohort and self-controlled case series analysis. BMJ (Clinical research ed). 2022;376:e068373. [DOI] [PMC free article] [PubMed]
- 30.Ministry of Health Malaysia. Health Facts 2019. 2019.
- 31.Hwong W.Y., Ang S.H., Bots M.L., Sivasampu S., Selvarajah S., Law W.C., et al. Trends of Stroke Incidence and 28-Day All-Cause Mortality after a Stroke in Malaysia: A Linkage of National Data Sources. Global Heart. 2021;16(1) doi: 10.5334/gh.79110.5334/gh.791.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forman R., Jit M., Mossialos E. Divergent vaccination policies could fuel mistrust and hesitancy. Lancet (London, England) 2021;397(10292):2333. doi: 10.1016/S0140-6736(21)01106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ministry of Health Malaysia. Clinical guidelines on COVID-19 vaccination in Malaysia (4th Edition). Malaysia; 2021.
- 34.Husby A, Hansen JV, Fosbøl E, Thiesson EM, Madsen M, Thomsen RW, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ (Clinical research ed). 2021;375:e068665. [DOI] [PMC free article] [PubMed]
- 35.Kochhar S., Excler J.-L., Bok K., Gurwith M., McNeil M.M., Seligman S.J., et al. Defining the interval for monitoring potential adverse events following immunization (AEFIs) after receipt of live viral vectored vaccines. Vaccine. 2019;37(38):5796–5802. doi: 10.1016/j.vaccine.2018.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suah J.L., Tok P.S.K., Ong S.M., Husin M., Tng B.H., Sivasampu S., et al. PICK-ing Malaysia's Epidemic Apart: Effectiveness of a Diverse COVID-19 Vaccine Portfolio. Vaccines. 2021;9(12):1381. doi: 10.3390/vaccines9121381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available within the article and its supplementary materials. Access to datasets is provided by the corresponding data custodians for analysis for this study, but we have no permission to make generated datasets available. Malaysia COVID-19 vaccine administration data are available at https://github.com/MoH-Malaysia/covid19-public, redacted of personal identifying information.