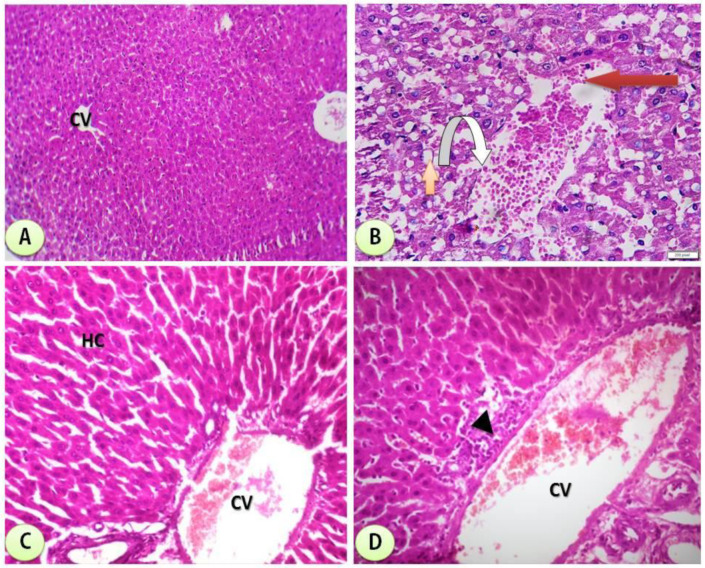
Figure 10.
(A) Control group: photomicrograph of cross-section of the hepatic tissues showing normal hepatic structure (H&Ex200). (B) STG group showing cross-section of experimental rat liver showing some adverse effects of STG administration on the liver comprising loss of normal architecture of liver lobules, ballooning degeneration of hepatocytes with cytoplasmic clearing (steatohepatitis) (orange arrow) and severe dilatation of the central vein with marked hemorrhage within it (red arrow) communicating with some dilated congested blood sinusoids (inverted arrow) (H&EX400). (C) STG/Mn-treated group showing amelioration of hepatic tissues with mildly dilated central vein (CV) at the center of the lobule surrounded by normal hepatocytes (HC) (H&Ex200). (D) STG/Co-treated group showing almost normal hepatic structure with dilated central vein (CV) at the center of the lobule surrounded by the hepatocytes; pericentral zone hepatocytes shows some necrosis (arrows) with lymphocytic inflammatory cell infiltrate (arrow head) H&Ex200).