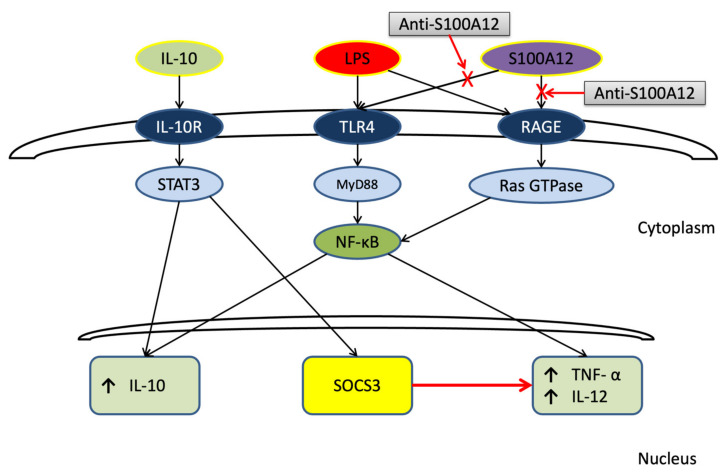
Figure 3.
A proposed schematic mechanism that shows S100A12 may modulate tumor necrosis factor (TNF)-α and interleukin (IL)-10 production through lipopolysaccharide (LPS). LPS links Toll-like receptor (TLR) 4 to activate nuclear factor-kappa B (NF-κB) through myeloid differentiation primary response 88 (MYD88). The activated NF-κB in the cytoplasm is then translocated into the nucleus where it binds to specific sequences of DNA and increases IL-10, IL-12, and TNF-α gene expressions. Extracellular IL-10 binds to IL-10 receptors and activates signal transducer and activator of transcription (STAT)3. Then, STAT3 is translocated to the cell nucleus and induces IL-10 gene expression. Activating STAT3 signaling also induces suppressor of cytokine signaling (SOCS)3 to suppress IL-12 and TNF-α gene expression. Once RAGE is bound to LPS or S100A12, NF-κB is activated by the active form of rat sarcoma (Ras), Ras nucleotide guanosine triphosphate (GTP) hydrolases (GTPase). S100A12 may competitively bind to TLR4/RAGE and the affinity between S100A12 and TLR4/RAGE may be lower than that between LPS and TLR/4RAGE. This results in increased IL-10 and TNF-α production with S100A12 being inhibited.