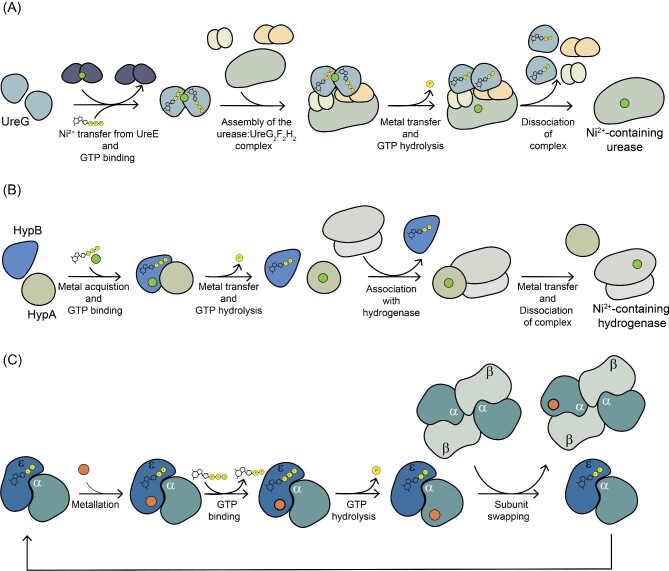
Fig. 3.
Postulated roles of G3E P-loop GTPases in metalloenzyme maturation. (A) Urease (pale green) requires the GTPase metallochaperone UreG (light blue) for insertion of the required Ni2+ ion. UreG receives the Ni2+ ion from UreE (dark blue) and binds GTP. The GTP binding event allows for the assembly of the apo-urease: the UreG2F2H2 complex. GTP hydrolysis triggers a conformational change that facilitates metal transfer to urease. After GTP hydrolysis occurs, the complex dissociates, and the urease now contains the needed Ni2+ ion for a fully activated enzyme.14,19,39 (B) [NiFe]-hydrogenases (light gray) require the metallochaperone HypB (blue) for Ni2+ ion insertion. HypB binds GTP and acquires Ni2+, which is transferred to HypA (pale green). HypA then interacts with the inactive hydrogenase to transfer the metal to the active site of the [NiFe]-hydrogenases for activation.21,44,45,93,99 (C) Fe-type NHases maturation with Fe ions (orange)51,52 involves a COG0532 GTPase metallochaperone, called ε or Nha3 (blue), which forms a complex with the α subunit of NHase (gray). The holo α subunit of NHase is swapped for an apo α subunit of NHase from the NHase α2 β2 complex. The process is repeated to fully mature the Fe-type NHases using the activating protein Nha3.51