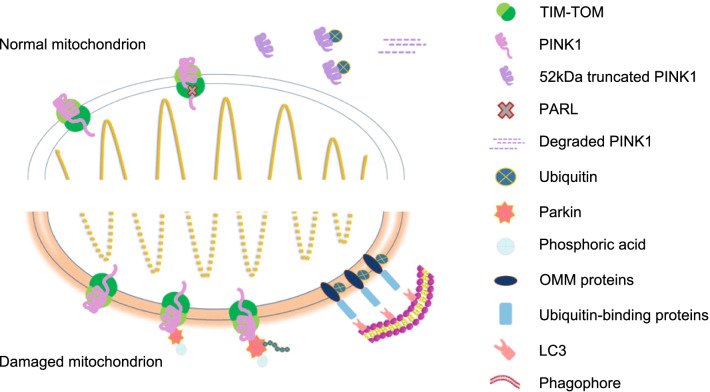
Fig. 1.
Ubiquitin-dependent mitophagy. PINK1 binds to the TOM complex and, under physiological conditions, is transported to the inner mitochondrial membrane and interacts with the TIM complex. PARL truncates the transmembrane segment of PINK1, releasing the 52-kDa ΔN-PINK1 into the cytoplasm. ΔN-PINK1 is then ubiquitylated and degraded by proteasomes. However, when mitochondria are damaged, PINK1 aggregates on the mitochondrial outer membrane and activates parkin. Parkin-dependent ubiquitin chains are then assembled on the outer membrane. Parkin ubiquitinates several mitochondrial outer membrane proteins, such as VDAC, Mfn2, and DRP1, which are then recognized by the ubiquitin-binding proteins, including OPTN, p62, NDP52, and NBR. These proteins recognize phosphorylated polyubiquitin chains and initiate autophagosome formation by binding to LC3. Finally, the damaged mitochondria recruit the autophagy pathway and fuse with the lysosome, leading to mitochondrial degradation