Abstract
Introduction
Diabetic ketoacidosis (DKA) is a complication of diabetes presenting with high anion gap metabolic acidosis. Methanol poisoning, on the other hand, is a toxicology emergency which presents with the same feature. We present a case of methanol poisoning who presented with DKA.
Case presentation
A 28-year-old male was referred to us with blurred vision and loss of consciousness three days after ingestion of 1.5 L of an unknown mixture of bootleg alcoholic beverage. He had history of insulin-dependent diabetes and had neglected his insulin shots on the day prior to hospital admission due to progressive loss of consciousness. Vital signs were normal and venous blood gas analysis showed severe metabolic acidosis and a methanol level of 10.2 mg/dL. After eight hours of hemodialysis, he remained unresponsive. Diabetic ketoacidosis was suspected due to positive urine ketone and blood sugar of 411 mg/dL. Insulin infusion was initiated which was followed by full awakening and extubation. He was discharged completely symptom-free after 4 weeks.
Conclusions
Diabetic ketoacidosis and methanol poisoning can happen simultaneously in a diabetic patient. Given the analogous high anion gap metabolic acidosis, physicians should pay particular attention to examination of the diabetic patients. Meticulous evaluation for both conditions is highly recommended.
Keywords: Methanol, Poisoning, Diabetic ketoacidosis
Introduction
Methanol intoxication is a toxicologic emergency which occurs due to ingestion of methanol-containing alcoholic beverages in countries where alcohol ingestion is prohibited. Since treatments should be initiated immediately in case of high suspicion, it is important to recognize methanol poisoning promptly [1]. Any delay in initiation of treatment results in detrimental consequences including blindness and death. Symptoms include gastrointestinal and visual complications that can lead to critical involvement of the central nervous system and eventually to complete vision loss. Formation of formic acid is responsible for the high anion gap metabolic acidosis (HAGMA) [2].
Diabetic ketoacidosis (DKA) is one of the most debilitating complications of diabetes presenting with nausea, vomiting, dehydration, abdominal pain, acetone breath, and Kussmaul breathing pattern [3]. Diabetic ketoacidosis is also associated with HAGMA. It is imperative to differentiate DKA from other causes of metabolic acidosis [4]. We present a case of methanol poisoning presenting with DKA.
Case presentation
A 28-year-old male presented to our emergency department with blurred vision and loss of consciousness three days after ingestion of 1.5 L of an unknown alcoholic beverage. He had a positive history of insulin-dependent diabetes mellitus and opium extract abuse. The patient’s companion declared that insulin shots had not been taken in the day prior to hospital admission. Physical examination revealed normal vital signs with a respiratory rate of 16 per minute. His first venous blood gas analysis (in the emergency department) showed severe metabolic acidosis with pH = 7.23, HCO3 = 7 mEq/L, BE = -19.1, pCO2 = 19 mmHg, and a methanol level of 10.2 mg/dL. Results of other lab tests are shown in Table 1.
Table 1.
On-presentation Lab tests of the patient (Normal range)
| Fasting blood sugar | 411 mg/dL (< 100 mg/dL) |
| Serum ketone | + |
| Creatinine | 3.1 mg/dL (0.59–1.04 mg/dL) |
| Urine drug testing | Opiates + |
| Urine analysis: | |
| pH | 5.5 |
| Color | Dark yellow |
| Appearance | Turbid |
| Blood | 3 + |
| Ketone | 1 + |
| Specific gravity | 1.026 |
| Bacteria | Many |
| Yeast cells | Many |
| Nitrite | Negative |
| WBCs | 25–30 |
| RBCs | Many |
| Urine culture | Candida |
| Serum lactate: | 31 mmol/L (0.5–2.2 mmol/L) |
| Bilirubin: | |
| Total | 1.8 mg/dL (0.1–1.2 mg/dL) |
| Direct | 0.5 mg/dL (< 0.3 mg/dL) |
| Serum Electrolytes: | |
| Na | 144 mEq/L (135–145 mEq/L) |
| K | 4.5 mEq/L (3,6–5.2 mEq/L) |
| Ca | 7 mg/dL (8.6–10.3 mg/dL) |
| P | 2.8 mg/dL (3.4–4.5 mg/dL) |
| Mg | 2.3 mg/dL (1.7–2.2 mg/dL) |
| Liver function tests: | |
| Ast | 196 U/L (10–40 U/L) |
| Alt | 257 U/L (7–56 U/L) |
| LDH | 2125 IU/L (140–280 IU/L) |
| CPK | 275 mg/dL (39–308 mg/dL) |
| Alkaline phosphatase | 382 IU/L (44–137 IU/L) |
| Complete blood count: | |
| White blood cells | 13.5 × 109/L (4.5–11 × 109/L) |
| Hemoglobulin | 16.2 mg/dL (13.2–16.6 mg/dL) |
| Platelet | 363 × 109/L (150–450 × 109/L) |
| ESR | 4 mm/hr (0–22 mm/hr) |
| CRP | 7.7 mg/dL (0.8–1 mg/dL) |
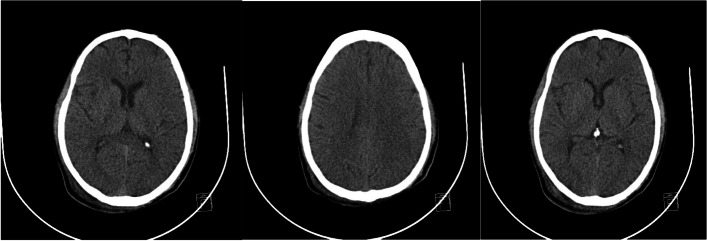
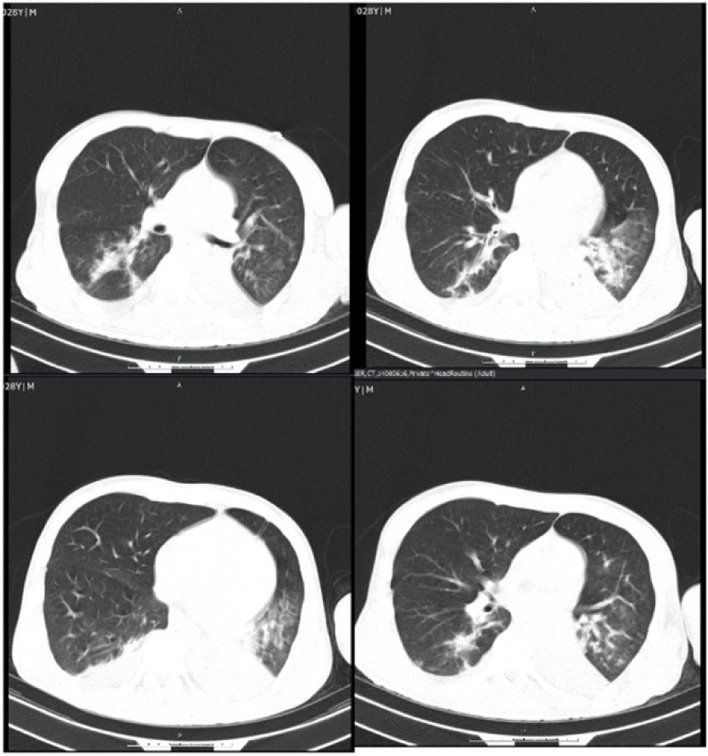
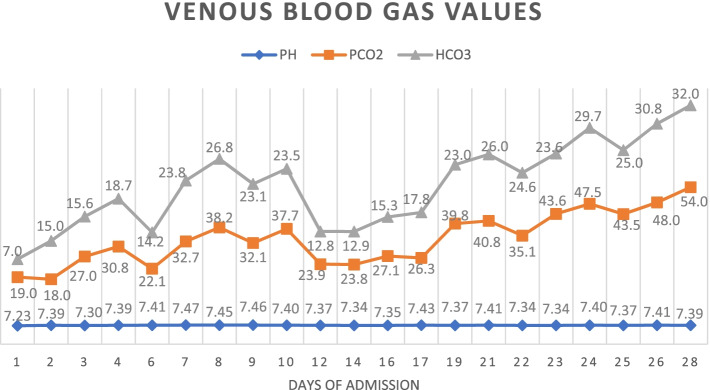
He was intubated and femoral catheterization was performed. Three vials of bicarbonate were intravenously administered. He underwent hemodialysis for eight hours but remained unresponsive. Serum/urine ketone and blood sugar were later returned to be positive and 411 mg/dL, respectively, indicating the possible presence of DKA. After initiation of insulin infusion, serum bicarbonate level started to rise. During admission, he had developed brain edema (Fig. 1). However, after appropriate treatment for DKA, the patient regained consciousness and was extubated. Since there were consolidations in his chest X-ray and lung computed tomography scan (Figs. 2 and 3), antibiotics and sepsis workup were ordered. He was initially treated by meropenem and vancomycin (for aspiration pneumonia); however, after consulting with the attending infectious disease specialist, he was started on levofloxacin. He was also receiving intravenous potassium, pantoprazole, heparin with prophylactic dose, and nebulized N-acetyl cysteine. Due to melena, endo-colonoscopy was performed but was reported to be normal. The other complication our patient experienced was deep venous thrombosis at the site of femoral catheter which mandated anticoagulation therapy with heparin drip. Diabetic ketoacidosis re-occurred twice more during the hospitalization. With the legitimate dose of methadone prescribed by psychiatrics, DKA completely resolved, and he was discharged home completely symptom-free after four weeks. Serial venous blood gas analysis, blood sugar and selected lab tests of the patient during hospital admission are shown in Figs. 4 and 5 and Table 2.
Fig. 1.
Brain computed tomography scan of the patient
Fig. 2.
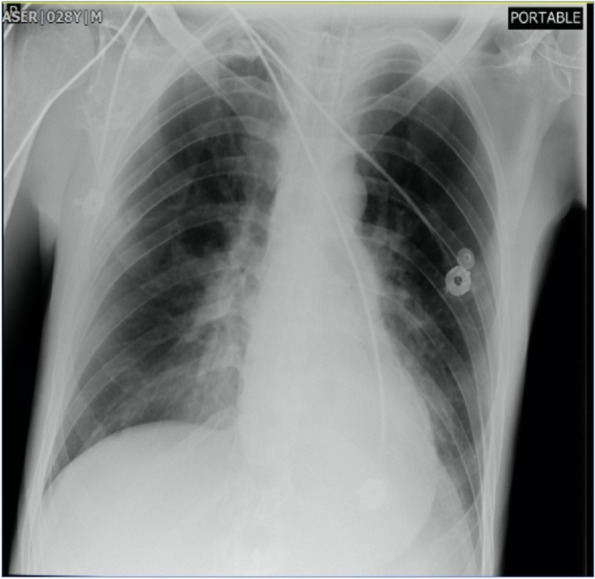
Chest X-ray of the patient
Fig. 3.
Lung computed tomography scan of the patient
Fig. 4.
Daily blood gas analysis during hospital stays
Fig. 5.
Fasting blood sugar changes during hospitalization
Table 2.
Serial lab test results (during the first five days of ICU admission and thereafter; Hemodialysis was performed before admission to ICU)
| Days of admission | |||||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 10th | 28th | |
| Creatinine (mg/dL) | 3.1 | 1.5 | 1.7 | 1.6 | 1.8 | 2 | 1.3 |
| K (mEq/L) | 4.5 | 4.4 | 3.5 | 3.0 | 3.9 | 3.6 | 4.5 |
| Na (mEq/L) | 130 | 137 | 140 | 141 | 146 | 142 | 138 |
| WBC (× 109 /L) | 13.5 | 8.6 | 7.6 | 7.6 | 8.9 | 9.3 | 7.2 |
| Hgb (mg/dL) | 16.2 | 13.3 | 12.1 | 12 | 11.3 | 11 | 11.2 |
| Platelet (× 109 /L) | 363 | 131 | 121 | 104 | 101 | 88 | 110 |
| Lactate (mg/dl) | 31 | - | 16 | - | 49 | 32 | 15 |
Discussion and conclusions
Alcoholic beverages are prohibited in Islamic countries like Iran; therefore, methanol intoxication is rather prevalent in these countries [5]. Methanol is essentially found in various household and industrial chemicals [6, 7]. Methanol poisoning often happens due to its accidental ingestion and can be fatal if left untreated. This intoxication should be included in differential diagnoses of any altered mental status [8].
In low-income countries, toxicologists face obstacles including lack of laboratory tests to detect blood levels of methanol and its metabolites, making the diagnosis and management more complicated [9, 10]. Methanol is not toxic per se, while accumulation of its active metabolite, formic acid, plays a major role in development of metabolic acidosis [11]. In the late stages of intoxication, cellular damage is due to acidosis and histotoxic effect of formate which is accompanied by formation of the free radicals [12].
Diabetic ketoacidosis is a life-threatening acute condition [13] which more commonly occurs in type I diabetes [14]. If DKA overlaps with HAGMA due to any other reason, it may become challengingly difficult to diagnose DKA.
In this case scenario, methanol poisoning was accompanied by DKA in a diabetic patient. Such condition pronounces the hyperglycemic effect of methanol. On the other hand, DKA itself can be due to excessive exogenous acids like methanol. Additionally, stress-induced hyperglycemia is seen in critically ill and poisoned patients. In other words, increases in counterregulatory hormones as a result of acute stress caused by poisoning may contribute to hyperglycemia [15]. A study by Sanaei-Zadeh et al. [16] showed that there was significant correlation between blood glucose level and blood pH suggesting that acidosis could be associated with hyperglycemia. Acute methanol poisoning is a physical stress provoking pancreatic injury and can trigger diabetes in susceptible patients [15, 17].
It is important to highlight that opium addiction may also lead to severe hyperglycemia and an increase in insulin resistance [18]. Current literature suggests a possible association of opium withdrawal with aggravation of hyperglycemia, as well, although the relation was not well clarified [19, 20]. Therefore, screening for opium consumption in patients with recurrent DKA may be reasonable and beneficial.
Diabetic ketoacidosis and methanol poisoning can happen simultaneously in a diabetic patient. Given the analogous HAGMA, physicians should pay particular attention to examination of the susceptible individuals. Meticulous evaluation for both conditions is highly recommended. Accurate follow-ups should also be emphasized.
Acknowledgements
None.
Abbreviation
- DKA
Diabetic ketoacidosis
Authors’ contributions
HHM, and NZ treated the patient. AE consulted the patient. MM and SS wrote the first manuscript. All authors were involved in the analysis and interpretation of findings. They proofread the manuscript and contributed for important intellectual content. All authors contributed to writing and approved the final manuscript.
Funding
The author(s) received no financial support for data collection, analysis, and interpretation of data or writing the manuscript.
Availability of data and materials
Data are available on request. To have access to the data please contact: Email: hassanian@sbmu.ac.ir, Tel: + 982,155,409,534.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the fundamental principles of the Declaration of Helsinki. The approval by the Research Ethics Committee of Shahid Beheshti University of Medical Sciences was waived because of the retrospective nature of case report.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editors-in-Chief of this journal.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barceloux DG, Randall Bond G, Krenzelok EP, Cooper H, Allister Vale J, American Academy of Clinical Toxicology Ad Hoc Committee on the Treatment Guidelines for Methanol Poisoning American academy of clinical toxicology practice guidelines on the treatment of methanol poisoning. J toxicol Clin Toxicol. 2002;40(4):415–46. doi: 10.1081/CLT-120006745. [DOI] [PubMed] [Google Scholar]
- 2.Sanaei-Zadeh H, Zamani N, Shadnia S. Outcomes of visual disturbances after methanol poisoning. Clin Toxicol. 2011;49(2):102–107. doi: 10.3109/15563650.2011.556642. [DOI] [PubMed] [Google Scholar]
- 3.Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician. 2013;87(5):337–346. [PubMed] [Google Scholar]
- 4.Kraut JA, Xing SX. Approach to the evaluation of a patient with an increased serum osmolar gap and high-anion-gap metabolic acidosis. Am J Kidney Dis. 2011;58(3):480–484. doi: 10.1053/j.ajkd.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Aghababaeian H, AraghiAhvazi L, Ostadtaghizadeh A. The methanol poisoning outbreaks in Iran 2018. Alcohol Alcohol. 2019;54(2):128–130. doi: 10.1093/alcalc/agz005. [DOI] [PubMed] [Google Scholar]
- 6.Tephly TR, McMartin KE. Methanol metabolism and toxicity. Aspartame-Physiology and Biochemistry. New York: Marcel Dekker; 1984: p 111–40.
- 7.Ng PC, Long BJ, Davis WT, Sessions DJ, Koyfman A. Toxic alcohol diagnosis and management: an emergency medicine review. Intern Emerg Med. 2018;13(3):375–383. doi: 10.1007/s11739-018-1799-9. [DOI] [PubMed] [Google Scholar]
- 8.Wiener SW. Toxic Alcohols. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, Eds. Goldfrank’s Toxicologic Emergencies, 10th edn. New York: McGraw-Hill; 2015. p. 1346–57.
- 9.Banagozar Mohammadi A, Delirrad M. Problems with methanol poisoning outbreaks in Iran. Alcohol Alcohol. 2019;54(3):338. doi: 10.1093/alcalc/agz028. [DOI] [PubMed] [Google Scholar]
- 10.Hassanian-Moghaddam H, Zamani N, Kolahi AA, McDonald R, Hovda KE. Double trouble: methanol outbreak in the wake of the COVID-19 pandemic in Iran-a cross-sectional assessment. Crit Care. 2020;24(1):402. doi: 10.1186/s13054-020-03140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMartin KE, Ambre JJ, Tephly TR. Methanol poisoning in human subjects: role for formic acid accumulation in the metabolic acidosis. Am J Med. 1980;68(3):414–418. doi: 10.1016/0002-9343(80)90113-8. [DOI] [PubMed] [Google Scholar]
- 12.Skrzydlewska E. Toxicological and metabolic consequences of methanol poisoning. Toxicol Mech Methods. 2003;13(4):277–293. doi: 10.1080/713857189. [DOI] [PubMed] [Google Scholar]
- 13.Celik U, Celik T, Avci A, Annagur A, Yilmaz HL, Kucukosmanoglu O, Topaloglu AK, Daglioglu N. Metabolic acidosis in a patient with type 1 diabetes mellitus complicated by methanol and amitriptyline intoxication. Eur J Emerg Med. 2009;16(1):45–48. doi: 10.1097/MEJ.0b013e3283034245. [DOI] [PubMed] [Google Scholar]
- 14.Mallare JT, Cordice CC, Ryan BA, Carey DE, Kreitzer PM, Frank GR. Identifying risk factors for the development of diabetic ketoacidosis in new onset type 1 diabetes mellitus. Clin Pediatr. 2003;42(7):591–597. doi: 10.1177/000992280304200704. [DOI] [PubMed] [Google Scholar]
- 15.Kajbaf F, Mojtahedzadeh M, Abdollahi M. Mechanisms underlying stress-induced hyperglycemia in critically ill patients. Clinical Practice. 2007;4(1):97. [Google Scholar]
- 16.Sanaei-Zadeh H, Esfeh SK, Zamani N, Jamshidi F, Shadnia S. Hyperglycemia is a strong prognostic factor of lethality in methanol poisoning. J Med Toxicol. 2011;7(3):189–194. doi: 10.1007/s13181-011-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shadnia S, Rahimi M, Soltaninejad K, Nilli A. Role of clinical and paraclinical manifestations of methanol poisoning in outcome prediction. J Res Med Sci. 2013;18(10):865. [PMC free article] [PubMed] [Google Scholar]
- 18.Mousavi SR, Dadpour B, Moshiri M, Moghiman T, Khosrojerdi H, Najari F. Methanol poisoning as a trigger for the presentation of diabetes mellitus: a case report. Int J Med Toxicol Forensic Med. 2019;9(3):151–154. doi: 10.32598/ijmtfm.v9i3.25085. [DOI] [Google Scholar]
- 19.Rezvanfar MR, Farahany H, Rafiee M, Kaboli S. Opium consumption challenge and diabetes mellitus control. Iran J Diabetes Obes. 2011;3(2):72–76. [Google Scholar]
- 20.Desser KB, Arvan S. Diabetic ketoacidosis during acute heroin abstinence. Lancet. 1969;2(7622):689–690. doi: 10.1016/S0140-6736(69)90392-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request. To have access to the data please contact: Email: hassanian@sbmu.ac.ir, Tel: + 982,155,409,534.