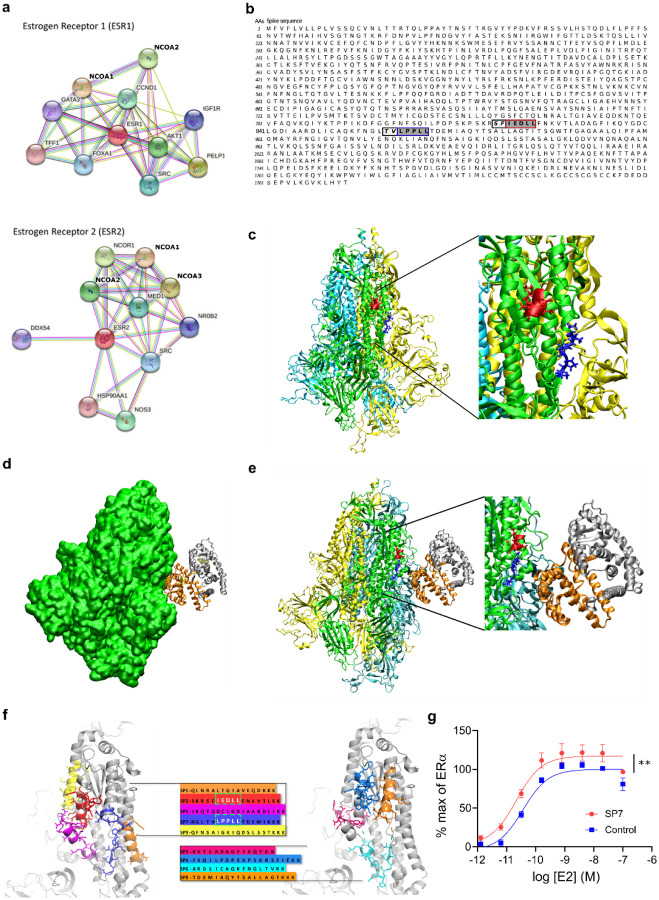
Figure 2. S and ER interact at conserved LXD nuclear receptor coregulatory (NRC) motifs.
(a) ER interaction network showing known and predicted protein associations. (b) LXD-like patterns in the S sequence. The LXXLL motif and a homologous region are highlighted in blue and red boxes, respectively, with dark grey background. −1 and −2 positions are reported in italic and light grey background. (c) The LPPLL and IEDLL residues of the two motifs are shown in the 3D X-ray S structure (pdb id 6VYB) with blue and red colors, respectively. The three S chains are shown in yellow, cyan and green. (d) S-ER motif-oriented docking. The best 3D docking hypothesis is shown. The ER dimer is in orange and gray, S is green. (e) Alignment between the best-pose and the 3OLL model tied with NCOA1. The region occupied by S’s alpha-helix interacts in the area where the NCOA fragment was crystallized. (f) S protein peptides and their location with respect to the S 3D structure. (g) The SP7 peptide containing the LPPLL motif significantly increased ERα activation (F (1,48) = 30.38; **P<0.01; two-way ANOVA, peptide treatment main effect). Data are mean ± SEM.