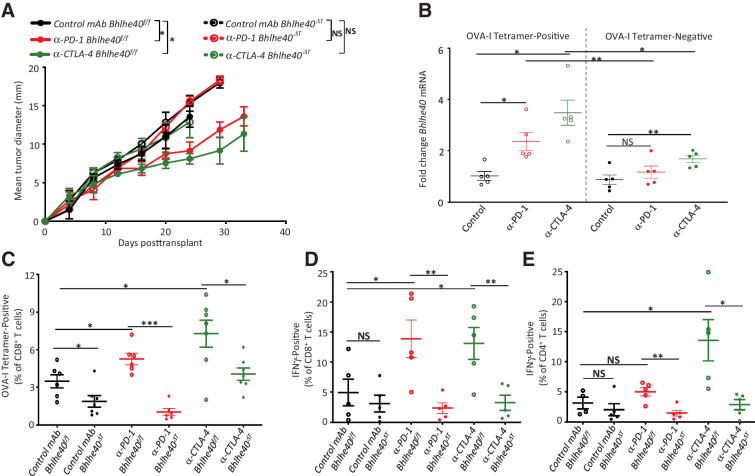
Figure 7.
Bhlhe40 is required for generation of functional tumor antigen–specific T cells and ICT efficacy against B16-OVA melanoma. A, B16-OVA tumor growth in Bhlhe40ΔT and Bhlhe40f/f mice treated with control mAb, anti–CTLA-4, or anti–PD-1. B,Bhlhe40 mRNA expression in intratumoral OVA-I tetramer–positive or –negative CD8+ T cells sorted from B16-OVA melanoma–bearing WT mice treated with control mAb, anti–CTLA-4, or anti–PD-1. C, Percent of intratumoral OVA tetramer–positive CD8+ T cells in B16-OVA melanoma–bearing Bhlhe40ΔT and Bhlhe40f/f mice treated as in B. Percent of intratumoral IFNγ-positive CD8+ T cells (stimulated ex vivo with OVA-I peptide; D) or CD4+ T cells (E; stimulated ex vivo with OVA-II peptide) in B16-OVA melanoma–bearing Bhlhe40ΔTor Bhlhe40f/f mice treated as in B. Data in A are presented as average tumor diameter ± SEM of 4–5 mice per group and are representative of at least 3 independent experiments (*, P < 0.05; **, P < 0.01, two-way ANOVA). Data in B are presented as mean ± SEM Bhlhe40 mRNA fold change. Each dot represents a Bhlhe40 mRNA data point from sorted OVA-I tetramer–positive and –negative CD8+ T cells isolated from 4–5 individual mice per group and are representative of at least 3 independent experiments. Data in D and E are presented as mean ± SEM of IFNγ+ cells expressed as a percent of CD8+ T cells or CD4+ T cells as assessed by flow cytometry. For C–E, cells were gated on live CD45+Thy1.2+ and CD8+ or CD4+ T cells. For B–E, cells were isolated from 4–5 individual mice per group on day 15 posttransplant (*, P < 0.05; **, P < 0.01; NS, not significant, unpaired t test).