Figure 4.
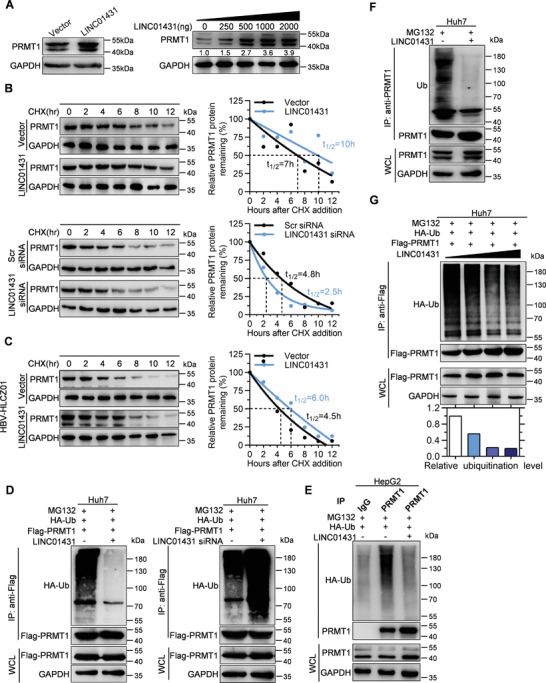
LINC01431 blocks the ubiquitination and degradation of PRMT1. A) Immunoblot analysis of PRMT1 in Huh7 cells transfected with LINC01431. B) Immunoblot analysis for the half‐life of PRMT1 in Huh7 cells transfected with LINC01431 or LINC01431 siRNA following treatment with the protein synthesis inhibitor CHX. C) Immunoblot analysis for the half‐life of PRMT1 in HBV‐infected HLCZ01 cells transfected with LINC01431 following treatment with CHX. D–G) Ubiquitination of PRMT1 in HCC cells. D) Ubiquitination of exogenous PRMT1 in Huh7 cells transfected with Flag‐PRMT1 and LINC01431 (left) or LINC01431 siRNA (right). E,F) Ubiquitination of endogenous PRMT1 in HepG2 cells (E) and Huh7 cells (F) transfected with LINC01431. G) Huh7 cells were transfected with Flag‐PRMT1 and an increased dose of LINC01431. Cells were cultured with the presence of MG132, and the ubiquitination of exogenous PRMT1 (D,G) or endogenous PRMT1 (E,F) was detected at day 3 post transfection by immunoprecipitating with anti‐Flag or anti‐PRMT1 antibodies, followed by immunoblotting with indicated antibodies. PRMT1 and GAPDH served as the loading control. CHX (400 µg mL−1) was used to inhibit the protein‐synthesis of PRMT1, and MG132 (20 µm) was added before protein extraction to inhibit PRMT1 degradation. The relative ubiquitination level was calculated as follows: relative ubiquitination level = band density ratio (ubiquitination band density / immunoprecipitated PRMT1 band density) (set as 1 at control group). For all experiments, representative of 3 independent experiments.
