Figure 4.
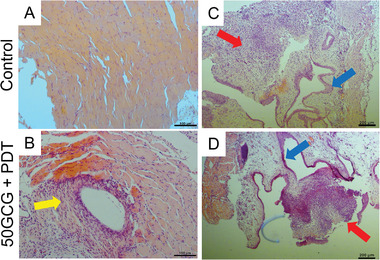
Biosafety evaluation of the implanted device and of photodynamic‐chemotherapy with glycosylated carrier. Mice were terminated after one PDT treatment (on day 18) 24 h after device activation. Hematoxylin and eosin staining of A) abdominal muscle from control mice and B) abdominal and fibrous tissue capsule surrounding the micro‐LED device in 50GCG + PDT treated mice. Objective lens, 10×. Scale bar, 100 µm. The yellow arrow indicates areas of increased nucleation in the fibrous tissue. C,D) Hematoxylin and eosin staining of bladder sections implanted with tumor. The red arrows indicate implanted tumor, and the blue arrows indicate healthy urothelium. Objective lens, 4×. Scale bar, 200 µm.
