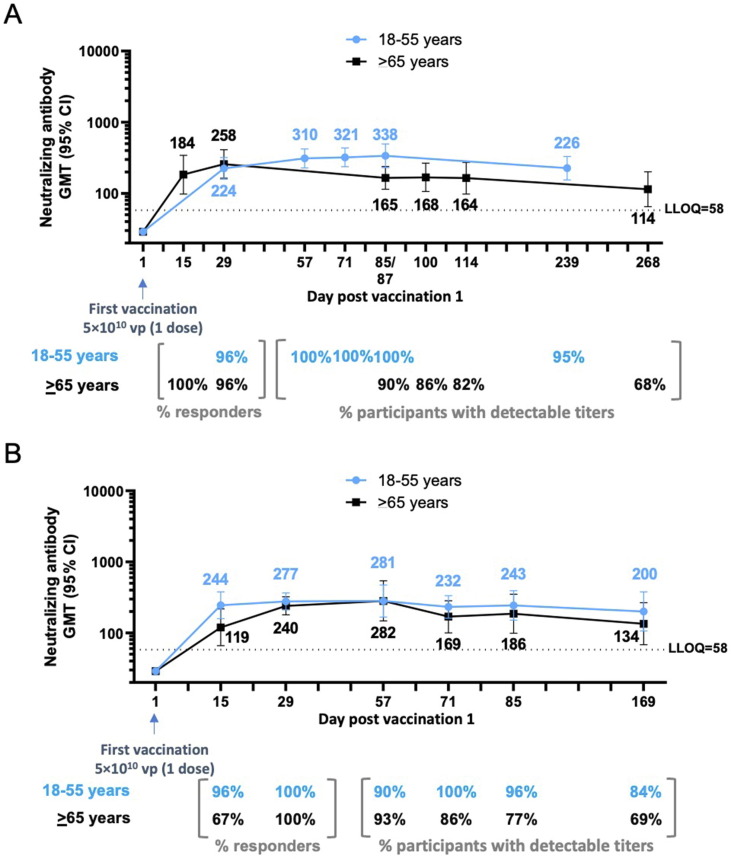
Fig. 3.
Durability of neutralizing antibody responses following a single dose of Ad26.COV2.S (5 × 1010 vp) (phase 1/2a and phase 2 trials). Phase 1/2a participants, aged 18–55 years (Cohort 1a) and aged ≥ 65 years (Cohort 3), were administered a single dose of Ad26.COV2.S (5 × 1010 vp) at Day 1. (A) Serum neutralizing antibody responses against SARS-CoV-2 were evaluated by wtVNA up to 8–9 months after the dose 1, in subsets of participants aged 18–55 years (N = 25; 22 with available data at Day 239; blue line) and ≥ 65 years (N = 24; 19 with available data at Day 268; black line). (B) Phase 2 participants, aged 18–55 years and ≥ 65 years, were administered a single dose of Ad26.COV2.S (5 × 1010 vp) at Day 1. Serum neutralizing antibody responses against SARS-CoV-2 were evaluated by wtVNA up to 6 months after dose 1 in subsets of participants aged 18–55 years (N = 22; blue line) and ≥ 65 years (N = 15; black line). GMTs are depicted above each time point, and response rates are illustrated at the bottom of each panel. In all panels, error bars represent 95% CIs. CI, confidence interval; GMT, geometric mean titer; LLOQ, lower limit of quantification; vp, viral particles; wtVNA, wild-type virus neutralization assay.