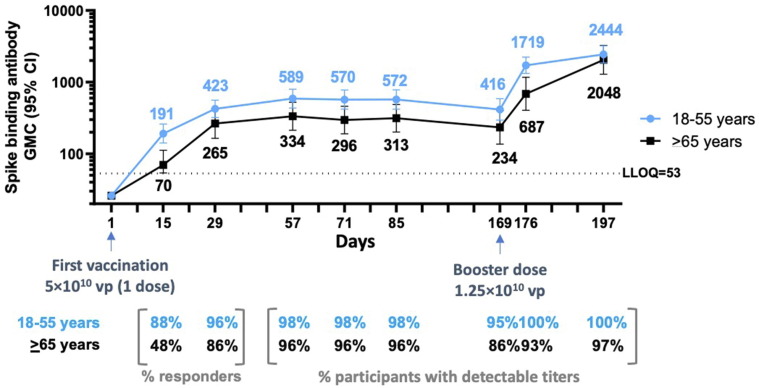
Fig. 5.
Durability of spike-binding antibody responses 6 months after first vaccination and responses 7 and 28 days after boosting (phase 2 trial). Phase 2 participants aged 18–55 years (N = 44; blue line) and ≥ 65 years (N = 29; black line) were administered a single dose of Ad26.COV2.S (5 × 1010 vp) at Day 1 and 73 received a booster dose of Ad26.COV2.S (1.25 × 1010 vp) at approximately 6 months (Day 169) after dose 1. Serum spike-binding antibody responses against SARS-CoV-2 were evaluated by validated S-ELISA up to 28 days after the booster dose (Day 197). GMCs are depicted above each time point (error bars represent 95% CIs), and response rates are illustrated at the bottom of each panel. CI, confidence interval; GMC, geometric mean concentration; LLOQ, lower limit of quantification; S-ELISA, spike protein enzyme-linked immunosorbent assay; vp, viral particles.