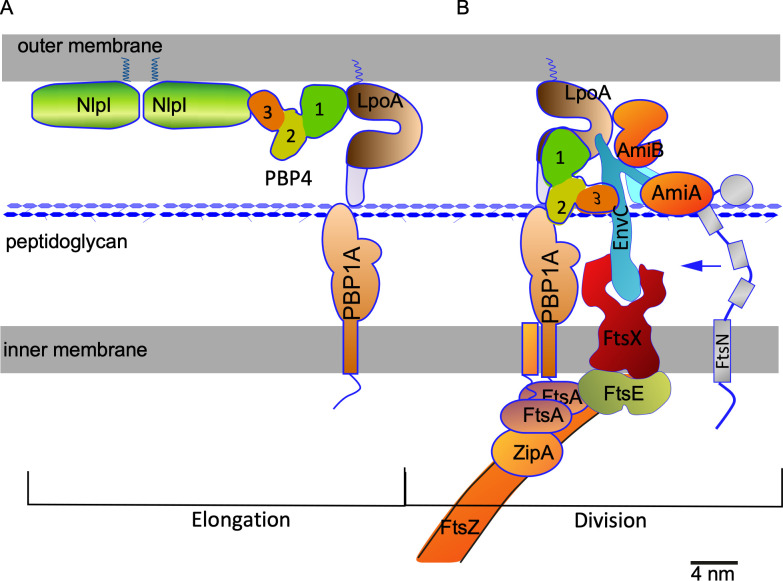
Fig 9. Model of the organization of PBP4 in the periplasm of E. coli.
(A) During elongation PBP4 (or a fraction of the PBP4 molecules) is kept away from its substrate through its interaction with NlpI and possibly LpoA. (B) PBP1A and LpoA associate with ZipA and the Z-ring to assist in preseptal PG synthesis. Because of their presence, the absence of NlpI and the newly synthesized PG, PBP4 is attracted by PBP1A and LpoA at the septal synthesis site, where it interacts with the FtsEXEnvC complex. Preseptal PG synthesis might provide substrate for PBP4 and the activated AmiA and B to produce denuded strand that attract FtsN. All proteins apart from the FtsZ polymer have been drawn approximately according to their crystal structure shape and hydrated crystal structure sizes (scale bar equals 4 nm). The membranes are assumed to be 2 nm and the distance between the outer and the inner membrane 21 nm.