Abstract
The recent description of biallelic DNAJC30 variants in Leber hereditary optic neuropathy (LHON) and Leigh syndrome challenged the longstanding assumption for LHON to be exclusively maternally inherited and broadened the genetic spectrum of Leigh syndrome, the most frequent paediatric mitochondrial disease. Herein, we characterize 28 so far unreported individuals from 26 families carrying a homozygous DNAJC30 p.Tyr51Cys founder variant, 24 manifesting with LHON, two manifesting with Leigh syndrome, and two remaining asymptomatic. This collection of unreported variant carriers confirms sex-dependent incomplete penetrance of the homozygous variant given a significant male predominance of disease and the report of asymptomatic homozygous variant carriers. The autosomal recessive LHON patients demonstrate an earlier age of disease onset and a higher rate of idebenone-treated and spontaneous recovery of vision in comparison to reported figures for maternally inherited disease. Moreover, the report of two additional patients with childhood- or adult-onset Leigh syndrome further evidences the association of DNAJC30 with Leigh syndrome, previously only reported in a single childhood-onset case.
Keywords: DNAJC30, mitochondrial disease, LHON, Leigh syndrome
Stenton et al. describe 28 individuals with LHON, Leigh syndrome or asymptomatic carriage of a pathogenic founder variant in the autosomal recessive mitochondrial disease gene DNAJC30. The findings confirm sex-dependent incomplete penetrance, frequent occurrence of recessive LHON, and the association of DNAJC30 with Leigh syndrome.
Introduction
Leber hereditary optic neuropathy (LHON) is due to selective retinal ganglion cell degeneration, causing rapid, bilateral, usually sequential, painless loss of central vision.1–4 LHON has an estimated minimum point prevalence of 3.22 per 100 000 in Europe5 and is the most frequent and clearly distinguishable mitochondrial disease. Spontaneous recovery of vision is rare.6–8 However, with the approval of idebenone by the European Medicines Agency, recovery rates are now reported up to 46%.7,9,10
Until recently, LHON was considered to be exclusively maternally inherited, with 95% of familial cases reported to be due to pathogenic variants in the mitochondrial genome (mtDNA) affecting subunits of mitochondrial respiratory chain complex I (NADH-ubiquinone oxidoreductase, RCCI).11 The description of pathogenic variants in the nuclear encoded gene DNAJC30, reported to both affect mitochondrial RCCI maintenance12 and to interact with the mitochondrial ATP-synthase (RCCV) machinery,13 largely bridged the diagnostic gap and led to the stratification of LHON into maternal LHON (mtLHON) and autosomal recessive LHON (arLHON). Specific pathogenic variants underpinning both mtLHON and arLHON demonstrate sex-dependent incomplete penetrance.12,14 In arLHON, these phenomena were associated with a rare homozygous Eastern European founder variant in DNAJC30 (NM_032317.2 c.152A>G, NP_115693.2 p.Tyr51Cys). This variant has a minor allele frequency of 0.12% in the gnomAD population, with no reported homozygous carriers, and results in near complete loss of DNAJC30 protein expression.12
To date, approximately 30 LHON patients carrying this variant in homozygosity have been identified,12 resulting in the stratification of LHON into arLHON and mtLHON. However, notably, in a single patient, the same pathogenic homozygous variant in DNAJC30 was reported to manifest with Leigh syndrome, a heterogenous neurodegenerative mitochondrial disease characterized by bilateral symmetrical lesions within the basal ganglia and brainstem structures.15
Herein, we characterize 28 so far unreported individuals carrying the homozygous p.Tyr51Cys DNAJC30 founder variant. Of these individuals, 24 manifested with LHON, two with Leigh syndrome and two remained asymptomatic.
Materials and methods
Study participants
LHON/Leigh syndrome patients and asymptomatic siblings harbouring the homozygous DNAJC30 p.Tyr51Cys variant were identified through routine clinical investigation of patients with suspected mitochondrial disease between January 2021 and December 2021. The study was approved by local ethical review boards and was performed under the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained for all participants.
Molecular genetic investigation
DNA was extracted from blood and DNAJC30 was sequenced by Sanger or exome sequencing. Carrier testing was offered to family members to confirm segregation and to screen for asymptomatic carriers.
Data collection from reported individuals
Reported data on 38 homozygous variant carriers (32 presenting with arLHON, one with Leigh syndrome and five remaining asymptomatic) were extracted from Stenton et al.12 Reported data on the age of onset of mtLHON were extracted from Rosenberg et al.16 (n = 104) and for visual acuity recovery rate in mtLHON with (n = 184) and without (n = 88) idebenone therapy from Catarino et al.,9 Klopstock et al.10 and Carelli et al.7
Analysis of visual acuity
Visual acuity was assessed using the logarithm of the minimal angle of resolution (logMAR). Clinically relevant recovery (CRR) of visual acuity was defined as improvement ≥0.2 logMAR or change from ‘off chart’ (logMAR > 1.68) to ‘on chart’, as previously established.9 The magnitude of visual acuity recovery was calculated as improvement in logMAR between nadir and last examination.
Statistical analysis
Descriptive statistical analyses were performed in R (version 4.1.0). Due to the rarity of the disease, no sample size calculation was performed in advance and the study was not powered for specific statistical hypotheses. Fisher’s exact tests were performed to compare the proportion of categorical variables between groups. Student’s t-tests or Mann-Whitney U-tests were performed to compare the mean of two groups for normally and non-normally distributed data, respectively. Kruskal-Wallis tests followed by post hoc Mann-Whitney U-tests were performed when comparing >2 groups. All statistical tests were two-sided, and significance was determined at <0.05.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Identification and characterization of unreported homozygous DNAJC30 p.Tyr51Cys variant carriers
Sanger or exome sequencing identified the homozygous DNAJC30 p.Tyr51Cys variant in a total of 24 unreported unrelated LHON patients (n = 22 male, n = 2 female), two unreported unrelated Leigh syndrome patients (n = 1 male, n = 1 female) and two unreported male asymptomatic siblings of affected probands. The variant carriers originated from Czech Republic (n = 15), Russia (n = 6), USA (n = 2), Romania (n = 1), Poland (n = 1), Turkey (n = 1), Sweden (n = 1) and Germany (n = 1).
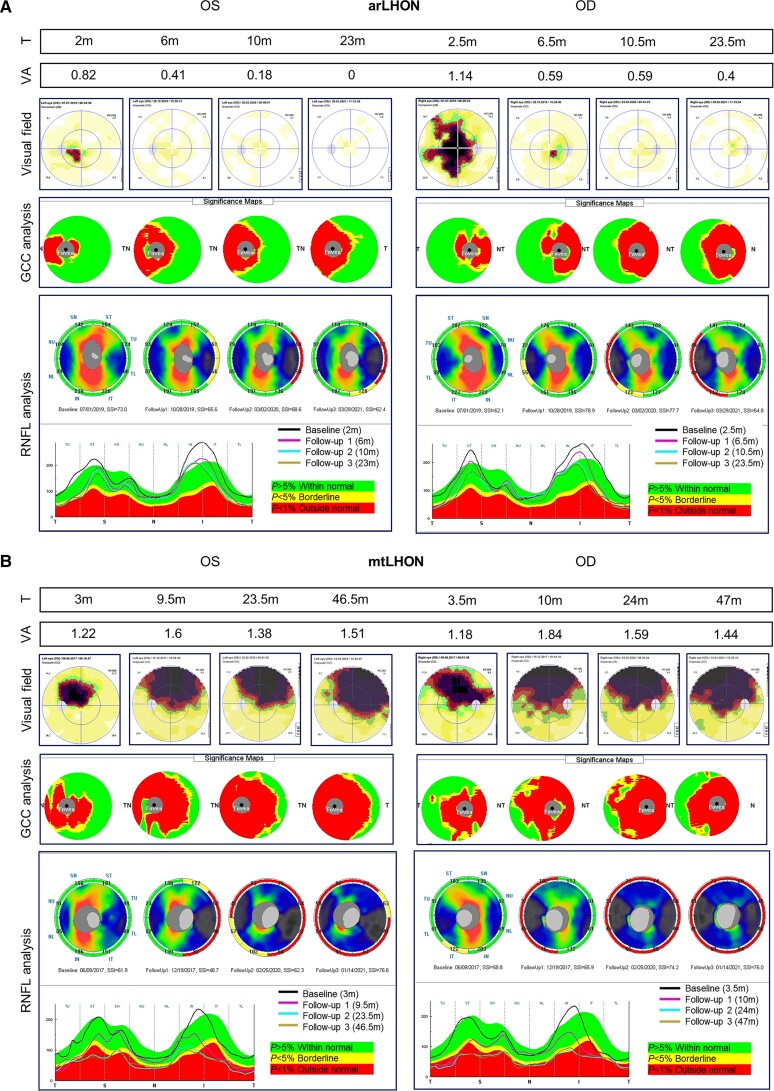
Clinicians reported the arLHON patients to be clinically indistinguishable from mtLHON. Patients with arLHON demonstrated rapid, bilateral, often sequential, painless loss of central vision. Where available, ophthalmological investigation revealed subacute phase swelling (pseudoedema) of the retinal nerve fibre layer, followed by chronic phase thinning, as exemplified for one arLHON patient in comparison to one mtLHON patient in Fig. 1. Mitochondrial dysfunction was reported in investigated arLHON patients (n = 2). These patients demonstrated a clear isolated mitochondrial RCCI defect in skeletal muscle tissue, with 0.18 mU/mUCS and 0.20 mU/mUCS residual RCCI activity, respectively (reference range 0.24–0.48 mU/mUCS).
Figure 1.
Ophthalmological investigation of arLHON and mtLHON. Illustrative example of (A) arLHON (DNAJC30, p.Tyr51Cys) and (B) mtLHON (MT-ND4, m.11778G>А) patients at first investigation and subsequent follow-up investigation. Time from symptom onset (T) is indicated in months (m). Visual acuity (VA) was assessed using the logMAR scale and demonstrates visual impairment in arLHON and mtLHON. The arLHON patient demonstrates subsequent complete (OS) or partial (OD) restoration of vision. Visual field was studied by perimetry (Low Vision Center program Octopus 900, Interzeag AG) and demonstrates bilateral central scotomas in arLHON and mtLHON as well as gradual decrease in size and an increase in light sensitivity in arLHON. Analysis of the thickness of the ganglion cell complex (GCC) and the peripapillary layer of the retinal nerve fibre layer (RNFL; RTVue-100 optical coherence tomography, Optovue) demonstrates marked thinning of the GCC and subacute phase swelling of the RNFL, followed by chronic phase thinning of the RNFL in mtLHON that is less pronounced in arLHON. The RNFL thickness graphs display the RNFL thickness values in micrometres in the temporal (T), superior (S), nasal (N) and inferior (I) sectors in the first and subsequent follow-up investigations (visits presented as black, pink, blue and brown curves). OD = ocular dextra (right eye); OS = ocular sinister (left eye); SSI = signal strength index.
Prevalence of arLHON within the clinically diagnosed LHON population
Across our diagnostic centres for LHON in Russia, Czech Republic, Italy, Sweden and Germany, 27% (28/105), 12% (13/109), 5% (5/108), 4% (2/54) and <1% (3/428) of genetically confirmed LHON families were accounted for by homozygous pathogenic variants in DNAJC30, respectively. The remainder of genetically confirmed LHON families were accounted for by pathogenic variants in the mitochondrial genome.
Incomplete penetrance and male predominance associated with the homozygous DNAJC30 p.Tyr51Cys variant
In combination with reported individuals, a total of 66 individuals carrying the DNAJC30 p.Tyr51Cys variant in homozygosity (n = 56 male, n = 10 female) were analysed, of which 56 manifested with arLHON (n = 52 male, n = 4 female), three manifested with Leigh syndrome (n = 1 male, n = 2 female) and seven were asymptomatic at the time of last assessment (n = 3 male, n = 4 female). These figures resulted in an overall penetrance estimate of 89% (95% CI = 80–95%), stratified into 95% (95% CI = 85–98%) in males and 60% (95% CI = 31–83%) in females, leading to a significant male predominance in affected individuals (9:1 affected male:female ratio; P = 0.008, Fisher’s exact test).
Autosomal recessive LHON has an earlier onset and higher recovery rate than mtLHON
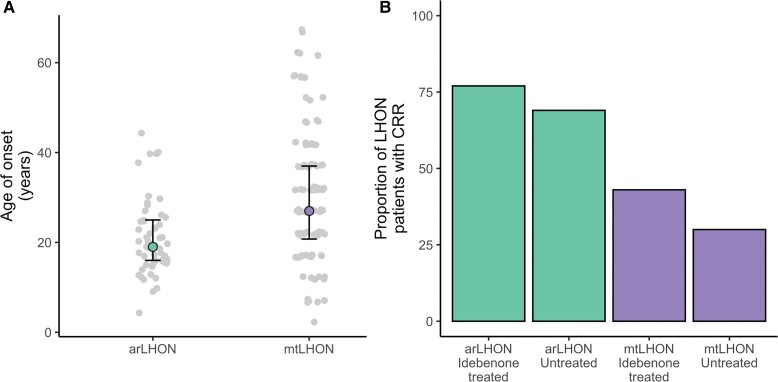
The median age of onset in arLHON was 19 years [range 9–44 years, interquartile range (IQR) 9 years]. In comparison to mtLHON patients (n = 104; median age of onset 27 years, range 2–67 years, IQR 16.25 years), the age of onset was significantly earlier (Mann-Whitney U-test, P = 2.5 × 10−5) and less variable (Fig. 2A). All arLHON patients reported bilateral involvement (112 affected eyes across 56 patients). Twenty-four arLHON patients experienced bilateral onset and 24 experienced sequential onset (data not available for eight patients). The median interval between eyes in patients with sequential onset was 12 weeks (range 1–48 weeks, IQR 13 weeks). The median time from onset to nadir was 10 weeks (range 0–77 weeks, IQR 12 months). At nadir, 29 of 112 eyes (26%) were off-chart, 56 eyes (50%) had a visual acuity 1.0–1.68 logMAR and four eyes (4%) had a visual acuity <1.0 logMAR (data not available for 23 eyes, 21%). Forty-six patients had follow-up visual acuity data for at least 6 months following onset (median follow-up time 3 years, range 0.5–19 years, IQR 5.75 years). Thirty-four of the 46 patients (74%; 66/92 eyes, 72%) experienced CRR from nadir. The magnitude of recovery of the patient’s best eye with CRR averaged 0.86 logMAR [standard deviation (SD) = 0.67], and in eight of the 34 patients experiencing CRR (24%), recovery was complete. Thirty of the 46 patients received idebenone therapy. The idebenone dose and duration of therapy were at the discretion of the treating physician. There was no significant difference in sex, age of onset or visual acuity at nadir between the treated (n = 30) and untreated (n = 16) patients (Table 1). Twenty-three of 30 idebenone-treated patients (77%) demonstrated CRR in at least one eye, a recovery rate significantly higher than reported for idebenone-treated mtLHON patients (80/184, 43%; P = 0.0008, Fisher’s exact test; Table 1 and Fig. 2B). Spontaneous CRR was reported in 11 of 16 untreated arLHON patients (69%), also significantly higher than reported for untreated mtLHON patients (26/88, 30%; P = 0.004, Fisher’s exact test). No difference in the mean magnitude of visual acuity improvement between the treated and untreated arLHON patients experiencing CRR was found (treated 1.1 logMAR versus untreated 1.16 logMAR, P = 0.72, Student’s t-test), and the 8% higher CRR rate in the treated arLHON patients did not reach significance, presumably due to lack of power (P = 0.73, Fisher’s exact test; Table 1). Our previous data do, however, demonstrate the time from nadir to recovery to be significantly shorted by idebenone therapy in arLHON.12 Notably, the follow-up time was substantially longer in the untreated patients demonstrating CRR (P-value = 0.0003, Kruskal-Wallis test; P-value = 0.00007, post hoc Mann Whitney U-test, idebenone treated recovery versus untreated recovery; Table 1).
Figure 2.
Comparison of disease onset and clinically relevant recovery rates of visual acuity in arLHON and mtLHON patients. (A) Reported age of onset for arLHON (n = 53, data unavailable for three patients) and mtLHON (n = 104). (B) Clinically relevant recovery rates for idebenone-treated (n = 30) and untreated (n = 16) arLHON patients (data presented for 46 of 56 arLHON patients with follow-up data available over at least 6 months following onset) and idebenone-treated (n = 184) and untreated (n = 88) mtLHON patients.
Table 1.
Clinical data of 46 idebenone-treated and untreated arLHON patients
| Idebenone-treated 30 patients (29 males), 60 eyes | Untreated 16 patients (14 males), 32 eyes | |||
|---|---|---|---|---|
| Recovery | No recovery | Recovery | No recovery | |
| Patients | 23 | 7 | 11 | 5 |
| Eyes | 46 | 14 | 22 | 10 |
| Age at onset in years | 19 (9–38) | 21 (15–29) | 20 (12–44) | 19 (12–40) |
| Interval of disease onset between eyes in weeks | 2.5 (0–32) | 12 (0–20) | 0 (0–48) | 0 (0–24) |
| VA at nadir in best eye | 1.4 (0.4–2.3) | 1.5 (1.1–1.7) | 1.7 (1–2) | 1 (0.6–2) |
| VA at nadir in worst eye | 1.4 (0.4–2.3) | 1.7 (1–2) | 1.4 (1.3–2) | 1.5 (1–2.3) |
| VA at last examination in best eye | 0.3 (0–1.7) | 1.5 (1.1–1.7) | 0.2 (0–1.3) | 1 (0.7–2.0) |
| VA at last examination in worst eye | 0.7 (0–1.9) | 1.7 (1–2) | 0.4 (0–1.3) | 1.2 (0.3–2.3) |
| Duration of follow-up from onset in years | 2 (0.5–11) | 1 (1–14) | 7 (1–13) | 6 (1–19) |
Data presented for 46 of the 56 identified arLHON patients with follow-up data available over at least 6-months following onset. Data are expressed as the median (range); disease onset refers to the first eye involved; visual acuity (VA) is expressed as logMAR and refers to the last examination.
DNAJC30-associated Leigh syndrome
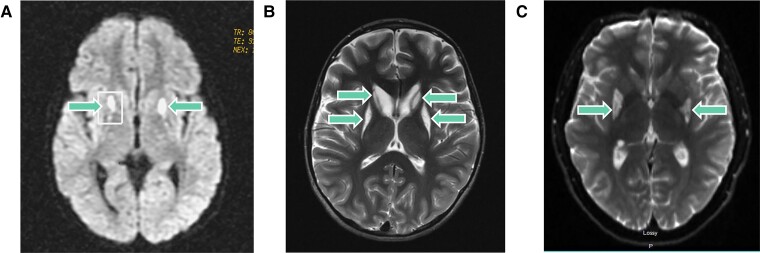
We previously reported one female patient (Patient 1) with childhood-onset Leigh syndrome in association with the homozygous DNAJC30 p.Tyr51Cys variant.12 Patient 1 presented at 4 years of age following a period of normal development with spasticity, progressive loss of gait and dysarthria. Her brain MRI revealed bilateral symmetrical lesions in the basal ganglia (Fig. 3A) and a lactate peak was reported with magnetic resonance spectroscopy (MRS). Serum and CSF lactate were normal. Measurement of mitochondrial RCC enzyme activities in skeletal muscle demonstrated an isolated RCCI defect (0.08 mU/mUCS, reference range 0.14–0.35). She is currently 24 years old, wheelchair-bound and experiencing severe spasticity and loss of motor skills with adequate cognitive development.
Figure 3.
MRI brain images from three patients with Leigh syndrome due to DNAJC30 defect. (A) MRI brain images from the first reported female childhood-onset Leigh syndrome patient (Patient 1) taken at 7 years of age demonstrating bilateral signal intensity changes in the putamina and the pedunculi cerebelli (arrows).(B) MRI brain images from the second reported female childhood-onset Leigh syndrome patient (Patient 2) taken at 12 years of age demonstrating bilateral signal intensity changes in the putamina and the heads of caudate nuclei (arrows). The volume of the putamina and caudate heads is decreased bilaterally. (C) MRI brain images from the male adult-onset DNAJC30-associated Leigh syndrome patient (Patient 3) taken at 24 years of age demonstrating bilateral signal intensity changes in the posterior basal ganglia (arrows).
Here, we report a second female childhood-onset Leigh syndrome patient (Patient 2) in addition to a male adult-onset Leigh syndrome patient (Patient 3). Patient 2 presented at 2 years of age following a period of normal development with strabismus. At 4 years of age, a severe upper respiratory tract infection triggered the development of a left-sided hemiplegia. Her brain MRI revealed bilateral symmetrical lesions involving the basal ganglia, brainstem and thalamus (Fig. 3B), and lactate peaks were reported within these lesions on brain MRS. Serum and CSF lactate were normal. Measurement of mitochondrial RCC enzyme activities in fibroblasts demonstrated an isolated RCCI defect (0.03 mU/mUCS, reference range 0.04–0.11); measurement in skeletal muscle was within normal range. She is currently 17 years old, experiencing loss of gait and speech, progressive dystonia and malnutrition necessitating a gastrostomy. Patient 3 presented at 19 years of age with an LHON/Leigh syndrome overlap syndrome, comprising acute visual loss, nystagmus, ophthalmoplegia, dysarthria and gait abnormalities. His MRI brain revealed bilateral symmetrical lesions in the basal ganglia and brainstem (Fig. 3C) and a lactate peak was reported on brain MRS. At 20 years of age, a febrile illness triggered worsening of his symptoms and the development of severe fatigue and central apnoea requiring respiratory support. Serum lactate was normal. He is currently 28 years old, independent in activities of daily living and experiencing chronic fatigue and a persistent dysarthria with normal cognitive function. None of the Leigh syndrome patients were treated with idebenone. Detailed case reports are available in the Supplementary material.
In explanation of the difference in phenotype between the Leigh syndrome and arLHON patients carrying the same homozygous DNAJC30 variant, screening for additional rare variants in DNAJC30 and in the potential protein-protein interaction partners of DNAJC30 (the mitochondrial RCCI and RCCV subunits) revealed all three Leigh syndrome patients to carry a rare heterozygous missense variant with high in silico pathogenicity prediction scores in a gene encoding a mitochondrial RCCI subunit: NDUFS8 c.305G>A (p.Arg102His) in Patient 1 (gnomAD minor allele frequency 0.002%, CADD 28.3, PolyPhen 0.99), NDUFS8 c.457T>C (p.Cys153Arg) in Patient 2 (gnomAD minor allele frequency 0.0004%, CADD 26.7, PolyPhen 1) and NDUFS2 c.980A>G (p.Tyr327Cys) in Patient 3 (absent from gnomAD, CADD 28.2, PolyPhen 0.99). In all three cases there was absence of a second rare variant to be in-keeping with the autosomal recessive mode of inheritance of these disease genes, thereby excluding them as the primary molecular genetic cause of the patient’s Leigh syndrome. Moreover, in Patient 1 whole genome sequencing, RNA sequencing and quantitative proteomics of the patient-derived fibroblast cell line were available for analysis. These methods did not reveal a second rare non-coding variant in NDUFS8 and demonstrated normal expression on both the RNA and protein level, thereby excluding NDUFS8 as the primary molecular genetic cause of the patient’s disease.
Discussion
Though autosomal recessive inheritance of LHON has been alluded to by single reports of recessive ‘LHON-like’ optic neuropathy due to pathogenic variants in NDUFS217 and MCAT,18 the identification, to date, of over 50 arLHON patients carrying variants in DNAJC30 argues for its frequent occurrence amongst patients with the distinctive clinical presentation of LHON. Indeed, based on the experience of our diagnostic centres for LHON we approximate the homozygous p.Tyr51Cys DNAJC30 variant to account for up to 27% of genetically diagnosed LHON families in the founder population of Eastern European and up to 5% of families in non-founder populations, demonstrating a gradient based on the distance from the geographical area of the founder event. The incomplete penetrance and resultant male predominance (9:1, male:female) associated with this variant have important implications for genetic counselling. Our estimation of penetrance in disease affected families does, however, result in a clinical ascertainment bias and is thereby likely to be an overestimation within the general population as families with only asymptomatic homozygous carriers would not come to medical attention for genetic sequencing. The stratification of LHON into recessively inherited (arLHON) and maternally inherited (mtLHON) disease has important implication for genetic and prognostic counselling due to the difference in mode of inheritance and demonstration of significant differences in age of disease onset and rate of visual recovery. The age of onset associated with arLHON is here confirmed to be earlier and more condensed than for mtLHON, with 50% of patients experiencing visual loss by 19 years of age. In contrast to the mostly permanent visual loss in mtLHON,19 our data suggest arLHON to have a better long-term prognosis with 77% of idebenone-treated and 69% of untreated patients experiencing clinically relevant visual recovery, and 17% of patients experiencing complete visual recovery in at least one eye. Although we were unable to confirm a statistically significant increase in recovery rate with idebenone treatment in arLHON, there was a trend towards this, with 8% higher recovery in the idebenone treated patients. This trend may be further unravelled in the future with larger patient numbers. Indeed, a power calculation based on these preliminary data indicate the need for enrolment of over 950 arLHON patients to achieve statistical power of 80%, (with 0.2 beta and 0.05 alpha), a substantial hurdle in the setting of rare disease research. Moreover, we were limited by the unavailability of data on treatment duration, known in mtLHON to influence the rate and magnitude of visual recovery,9 and the follow-up time in the untreated arLHON patients was substantially longer than for treated patients, allowing the patients more time to demonstrate visual recovery. Our previous data on a subset of the patients does, however, demonstrate the time from nadir to recovery to be significantly shorted by idebenone therapy in arLHON.12
The identification of a further two Leigh syndrome cases confirms the possibility for a more severe mitochondrial disease phenotype to arise in association with DNAJC30 variants with both childhood- and adult-onset. This phenomenon is also reported in association with certain pathogenic variants in the mitochondrial genome responsible for mtLHON.20,21 Notably, all three Leigh syndrome patients were alive at last report into the second and third decades of life, indicating long-survival Leigh syndrome to result from pathogenic variants in DNAJC30 in contrast to poorer survival reported in associated with other genetic aetiologies.22–26 Moreover, as Leigh syndrome does not typically manifest with acute visual loss, the identification of a patient with Leigh syndrome and acute visual loss demonstrates a potential LHON/Leigh syndrome overlap syndrome, as is also reported in association with certain rare mtDNA mutations in the subunits of mitochondrial RCCI responsible for mtLHON.27 Unfortunately, further ophthalmological investigations were not available for evaluation in this patient to clarify the association. In search of an explanation for the discrepancy in phenotype expression of the DNAJC30 variant in the arLHON and Leigh syndrome patients, we identified all three Leigh syndrome patients to carry an additional mitochondrial RCC1 subunit missense variant with high in silico pathogenicity prediction scores. These variants may contribute to the severity of the clinical presentation given their potential involvement in the mechanism of disease, impaired RCCI repair resulting in mitochondrial RCCI dysfunction.12 Published functional data to date, however, does not indicate more severe mitochondrial dysfunction in DNAJC30-associated Leigh syndrome in comparison to arLHON, investigated in one patient by measurement of mitochondrial RCCI activity in patient-derived muscle tissue and both mitochondrial RCCI dependent respiration rate and mitochondrial RCCI subunit repair in patient-derived fibroblasts.12 This phenomenon is well documented for RCCI dysfunction, with often times limited to no correlation between the residual RCCI activity and the clinical severity of disease.28
In conclusion, our data underline the diagnostic uplift of sequencing DNAJC30 in parallel with the mitochondrial DNA in sporadic clinically suspected LHON and the importance to consider DNAJC30 in the molecular diagnosis of Leigh syndrome. The confirmation of sex-dependent incomplete penetrance in our extended collection of variant carriers and the report of high visual recovery rates in arLHON are essential to family and prognostic counselling.
Supplementary Material
Acknowledgements
The National Center for Medical Genomics (LM2018132) provided instrumental and technical support with the exome sequencing analysis of the Czech families. The Swedish group (M.E. and F.T.) has received financial support from the Swedish patient organization LHON Eye Society. We would like to thank Leonardo Caporali for running diagnostic tests and Michele Carbonelli for running ophthalmologic examinations at the Italian diagnostic centre.
Glossary
Abbreviations
- arLHON
autosomal recessive LHON
- CRR
clinically relevant recovery
- LHON
Leber hereditary optic neuropathy
- logMAR
logarithm of the minimal angle of resolution
- mtLHON
maternal LHON
- RCC
respiratory chain complex
Funding
German Federal Ministry of Education and Research (BMBF, Bonn, Germany) and Horizon2020 through the E-Rare project GENOMIT (01GM1920A to HP, 01GM1920B to T.K., genomit.eu). BMBF grant to the German Network for Mitochondrial Disorders (mitoNET, 01GM1906D to H.P. and 01GM1906A to T.K.). Ricerca Corrente funding from the Italian Ministry of Health (to V.C. and C.L.M.). Charles University (Progress Q26/LF1 to B.K., UNCE/MED/007 to L.N., H.K.) and the Ministry of Health of the Czech Republic (RVOVFN64165 to M.T. and I.T., AZV NV19-07-00137 to L.N. and V.S.). T.K. and V.C. are members of the European Reference Network for Rare Neurological Diseases and of the European Reference Network for neuromuscular diseases (EURO-NMD), co-funded by the European Commission.
Competing interests
T.K., C.L.M. and V.C. have received research support, speaker honoraria, consulting fees, and travel reimbursement from Santhera Pharmaceuticals, Chiesi GmbH and GenSight Biologics. All other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Carelli V, Carbonelli M, Irenaeus F, et al. . International consensus statement on the clinical and therapeutic management of Leber hereditary optic neuropathy. J Neuroophthalmol. 2017;37(4):371–381. [DOI] [PubMed] [Google Scholar]
- 2. Newman NJ. Treatment of hereditary optic neuropathies. Nat Rev Neurol. 2012;8(10):545–556. [DOI] [PubMed] [Google Scholar]
- 3. Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies—Disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30(2):81–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barboni P, Savini G, Valentino ML, et al. . Leber’s hereditary optic neuropathy with childhood onset. Invest Ophthalmol Vis Sci. 2006;47(12):5303–5309. [DOI] [PubMed] [Google Scholar]
- 5. Man PYW, Griffiths PG, Brown DT, Howell N, Turnbull DM, Chinnery PF. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am J Hum Genet. 2003;72(2):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu-Wai-Man P, Votruba M, Moore AT, Chinnery PF. Treatment strategies for inherited optic neuropathies: past, present and future. Eye (Lond). 2014;28(5):521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carelli V, La Morgia C, Valentino ML, et al. . Idebenone treatment in Leber’s hereditary optic neuropathy. Brain. 2011;134(9):e188. [DOI] [PubMed] [Google Scholar]
- 8. Mashima Y, Kigasawa K, Wakakura M, Oguchi Y. Do idebenone and vitamin therapy shorten the time to achieve visual recovery in Leber hereditary optic neuropathy? J Neuroophthalmol. 2000;20(3):166–170. [DOI] [PubMed] [Google Scholar]
- 9. Catarino CB, von Livonius B, Priglinger C, et al. . Real-world clinical experience with idebenone in the treatment of leber hereditary optic neuropathy. J Neuroophthalmol. 2020;40(4):558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klopstock T, Yu-Wai-Man P, Dimitriadis K, et al. . A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011;134(9):2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mackey DA, Oostra RJ, Rosenberg T, et al. . Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet. 1996;59(2):481–485. [PMC free article] [PubMed] [Google Scholar]
- 12. Stenton SL, Sheremet NL, Catarino CB, et al. . Impaired complex I repair causes recessive Leber’s hereditary optic neuropathy. J Clin Invest. 2021;131(6):e138267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tebbenkamp AT, Varela L, Choi J, et al. . The 7q11.23 protein DNAJC30 interacts with ATP synthase and links mitochondria to brain development. Cell. 2018;175(4):1088–1104.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu-Wai-Man P, Votruba M, Burté F, La Morgia C, Barboni P, Carelli V. A neurodegenerative perspective on mitochondrial optic neuropathies. Acta Neuropathol. 2016;132(6):789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lake NJ, Bird MJ, Isohanni P, Paetau A. Leigh syndrome: neuropathology and pathogenesis. J Neuropathol Exp Neurol. 2015;74(6):482–492. [DOI] [PubMed] [Google Scholar]
- 16. Rosenberg T, Nørby S, Schwartz M, et al. . Prevalence and genetics of Leber hereditary optic neuropathy in the Danish population. Invest Ophthalmol Vis Sci. 2016;57(3):1370–1375. [DOI] [PubMed] [Google Scholar]
- 17. Gerber S, Ding MG, Gérard X, et al. . Compound heterozygosity for severe and hypomorphic NDUFS2 mutations cause non-syndromic LHON-like optic neuropathy. J Med Genet. 2017;54(5):346–356. [DOI] [PubMed] [Google Scholar]
- 18. Gerber S, Orssaud C, Kaplan J, Johansson C, Rozet JM. MCAT mutations cause nuclear LHON-like optic neuropathy. Genes (Basel). 2021;12(4):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newman NJ, Carelli V, Taiel M, Yu-Wai-Man P. Visual outcomes in Leber hereditary optic neuropathy patients with the m. 11778G>A (MTND4) mitochondrial DNA mutation. J Neuroophthalmol. 2020;40(4):547–557. [DOI] [PubMed] [Google Scholar]
- 20. Fruhman G, Landsverk ML, Lotze TE, et al. . Atypical presentation of Leigh syndrome associated with a Leber hereditary optic neuropathy primary mitochondrial DNA mutation. Mol Genet Metab. 2011;103(2):153–160. [DOI] [PubMed] [Google Scholar]
- 21. Miyaue N, Yamanishi Y, Tada S, et al. . Repetitive brainstem lesions in mitochondrial DNA 11778G>A mutation of Leber hereditary optic neuropathy. eNeurologicalSci. 2019;14:74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim AZ, Ng YS, Blain A, et al. . The natural history of Leigh syndrome: a study of disease burden and progression. Ann Neurol. 2022;91(1):117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ardissone A, Bruno C, Diodato D, et al. . Clinical, imaging, biochemical and molecular features in Leigh syndrome: a study from the Italian network of mitochondrial diseases. Orphanet J Rare Dis. 2021;16(1):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogawa E, Fushimi T, Ogawa-Tominaga M, et al. . Mortality of Japanese patients with Leigh syndrome: effects of age at onset and genetic diagnosis. J Inherit Metab Dis. 2020;43(4):819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee JS, Yoo T, Lee M, et al. . Genetic heterogeneity in Leigh syndrome: highlighting treatable and novel genetic causes. Clin Genet. 2020;97(4):586–594. [DOI] [PubMed] [Google Scholar]
- 26. Sofou K, de Coo IF, Ostergaard E, et al. . Phenotype-genotype correlations in Leigh syndrome: new insights from a multicentre study of 96 patients. J Med Genet. 2018;55(1):21–27. [DOI] [PubMed] [Google Scholar]
- 27. Liolitsa D, Rahman S, Benton S, Carr LJ, Hanna MG. Is the mitochondrial complex I ND5 gene a hot-spot for MELAS causing mutations? Ann Neurol. 2003;53(1):128–132. [DOI] [PubMed] [Google Scholar]
- 28. Distelmaier F, Koopman WJ, van den Heuvel LP, et al. . Mitochondrial complex I deficiency: from organelle dysfunction to clinical disease. Brain. 2008;132(4):833–842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.