Figure 1. Strategies to minimize clinical risks of kidney biopsy and optimize a range of benefits for participants in the Kidney Precision Medicine Project.
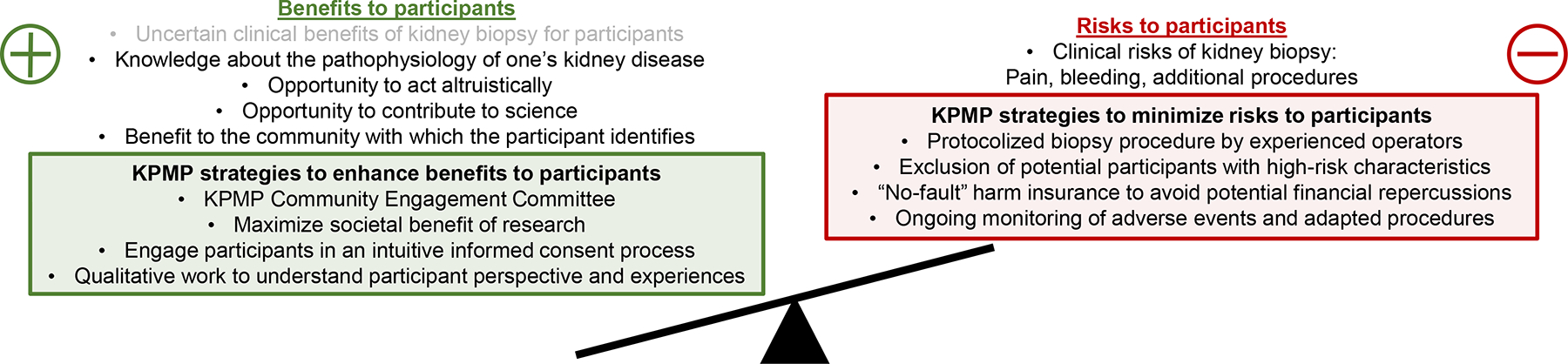
Research ethics as codified in the US Common Rule require that risks to research participants—such as risks associated with kidney biopsy as part of the Kidney Precision Medicine Project (KPMP)--are exceeded by potential benefits. Traditional clinical benefits of kidney biopsy are often equivocal for participants in KPMP, but appreciation of a range of participant-defined benefits reveals opportunities to enhance these benefits in the design and conduct of the KPMP.
