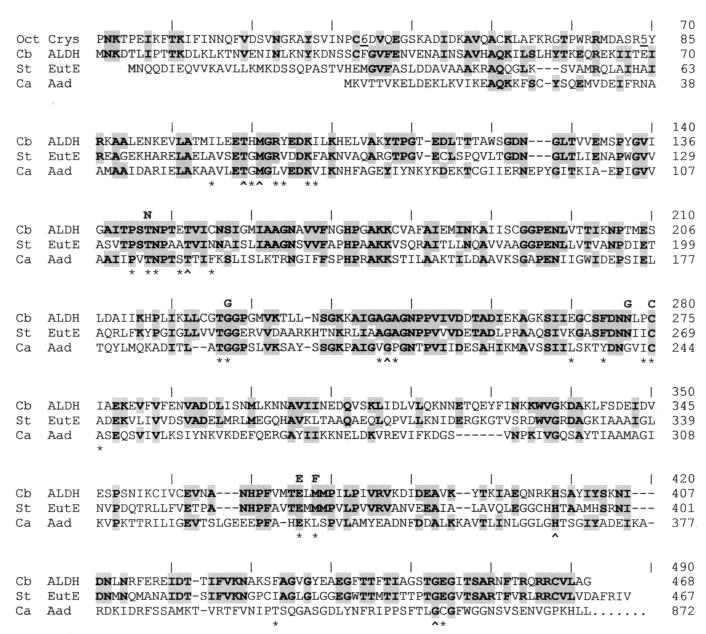
FIG. 4.
Alignment of the amino acid sequences of three CoA-dependent ALDHs and the N-terminal region of an ALDH-related protein. Markers above the sequences indicate the positions used in the alignment of the C. beijerinckii NRRL B593 ALDH. The sources and abbreviations are as follows: Oct Crys, Ω-crystallin of the giant octopus (41); Cb ALDH, ALDH of C. beijerinckii NRRL B593 (accession no. AF132754); St EutE, the eutE-encoded ALDH of S. typhimurium (AAA80209); Ca Aad, the ALDH domain encoded by the aad gene of C. acetobutylicum ATCC 824 (25). Amino acid residues that are shared by the C. beijerinckii NRRL B593 ALDH and at least one of the other sequences are highlighted. Positions of amino acids that line the proposed catalytic pocket of the rat ALDH3 are indicated by an asterisk or a caret (the latter indicates a residue that is conserved in nine CoA-dependent ALDHs but not in CoA-independent ALDHs at these positions). An underlined number in a sequence indicates the number of amino acids omitted at that position to save space. Dashes in the sequence indicate the gaps that were introduced to refine the alignment. Boldfaced letters above the sequences indicate amino acid residues conserved in previously aligned ALDHs with demonstrated dehydrogenase activity (28).