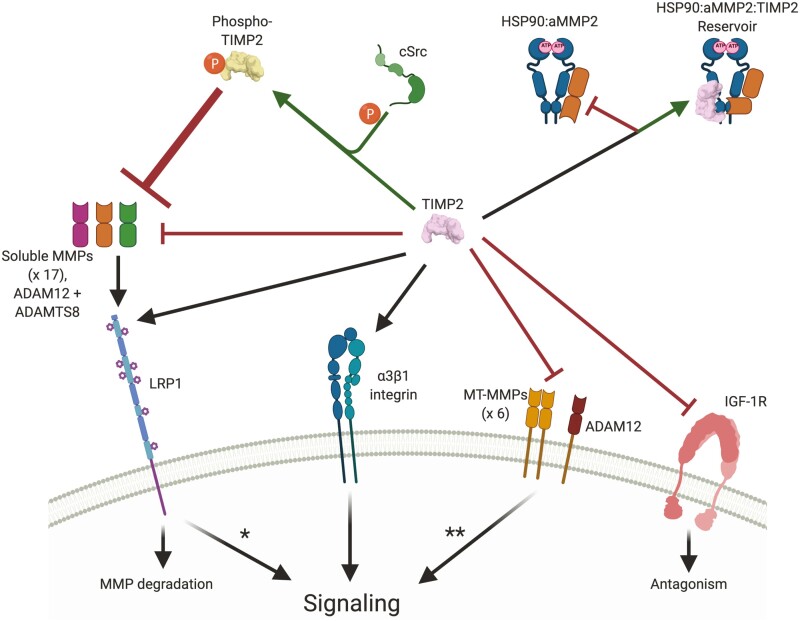
Figure 6.
The simplified TIMP2 interactome.
The major interacting partners for TIMP2 are the Metzincin family of proteinases (specifically, MMPs, ADAM12 and ADAMTS8). This proteinase inhibitory interaction can be positively modified by phosphorylation at tyrosine 90 by extracellular cSrc kinase. Active MMP2 (aMMP2) is stabilized by dimerization with extracellular HSP90, an interaction that can be disrupted by TIMP2. HSP90:aMMP2:TIMP2 can exists in a transient, catalytically inactive state that may act as a reservoir of rapidly available aMMP2. Both MMPs and TIMP2 are endocytosed by LRP1, which occurs with higher affinity when these are in an inhibitory complex. This results in MMP degradation and a mixture of degradation/recycling with regards to TIMP2. In addition, this interaction may result in the activation of intracellular signaling events. Furthermore, membrane-type MMPs and ADAM12 (ADAM12-L isoform) exist in a membrane-associated state and are actively targeted by TIMP2. This interaction can promote downstream signaling and endocytosis of the complex. Separate from TIMP2s proteinase-inhibitory capabilities, TIMP2 has been shown to mediate signaling through α3β1 integrin to promote cell signaling that are largely associated with indirect antagonism of tyrosine kinase receptors (see Figure 2). On the contrary, TIMP2 displays direct antagonism at the IGF-1 receptor (IGF-1R) through a region within its C-terminal domain. It remains to be seen how TIMP2 in complex with pro-MMP2 or specific active MMPs may affect the formation of this multitude of complexes. *Shown for TIMP1:LRP1 interaction only. **Shown for the TIMP2:MMP14 interaction only. Created with BioRender.com.