Figure 1.
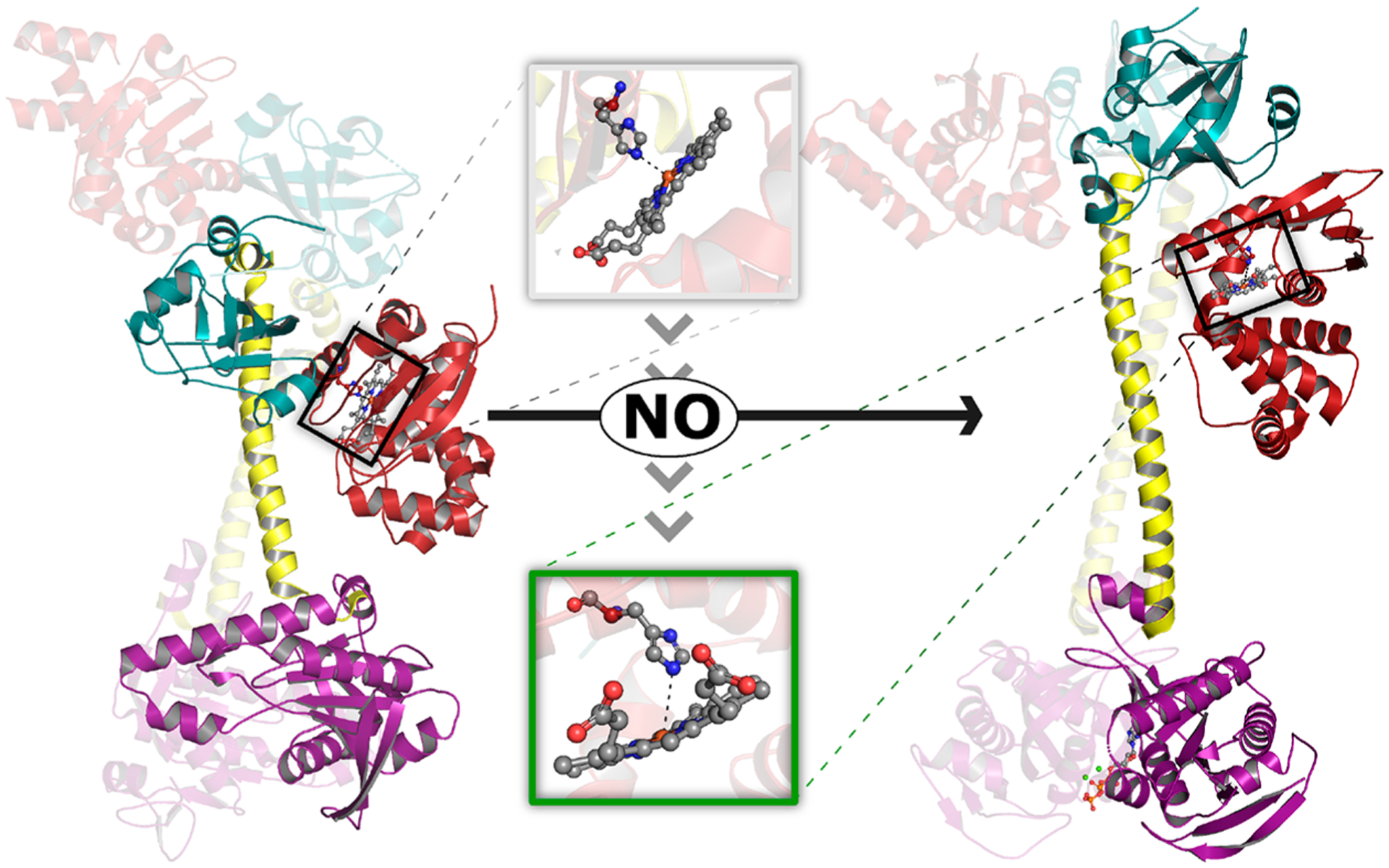
NO-induced structural rearrangement in sGC. Structural models depicting inactive (left, PDB 6JT1) and active NO-bound (right, PDB 6JT2) sGC were determined using cryo-EM.30 While the entire heterodimer is depicted for each model, the heme-containing β subunit is highlighted for clarity. Binding of NO to ferrous heme in the β-HNOB domain (red) induces a coil-to-helix transition that extends and reorients the central helices of the CC (yellow). CC elongation is accompanied by rotation of the β-HNOB and core HNOBA (cyan) domains away from the catalytic cyclase domain (purple). Together, these structural changes cause rotation of the relative orientation of the two cyclase domains, increasing the volume of the active site and allowing two Mg2+ cations (green spheres) and one GTP molecule (orange ball and stick) to bind at the interface of the catalytic domains. Inset: NO-induced structural changes at the sGC heme. NO binding brings the Fe atom back into the heme plane and is accompanied by a ~0.7 Å increase in the distance between the heme Fe atom and Nε2 atom of His105. We note that the resolution of the structural data (3.9 and 3.8 Å for inactive and active structures, respectively) precludes in-depth analysis of the heme coordination environment (e.g., the authors do not include heme-bound NO in the active structural model).
