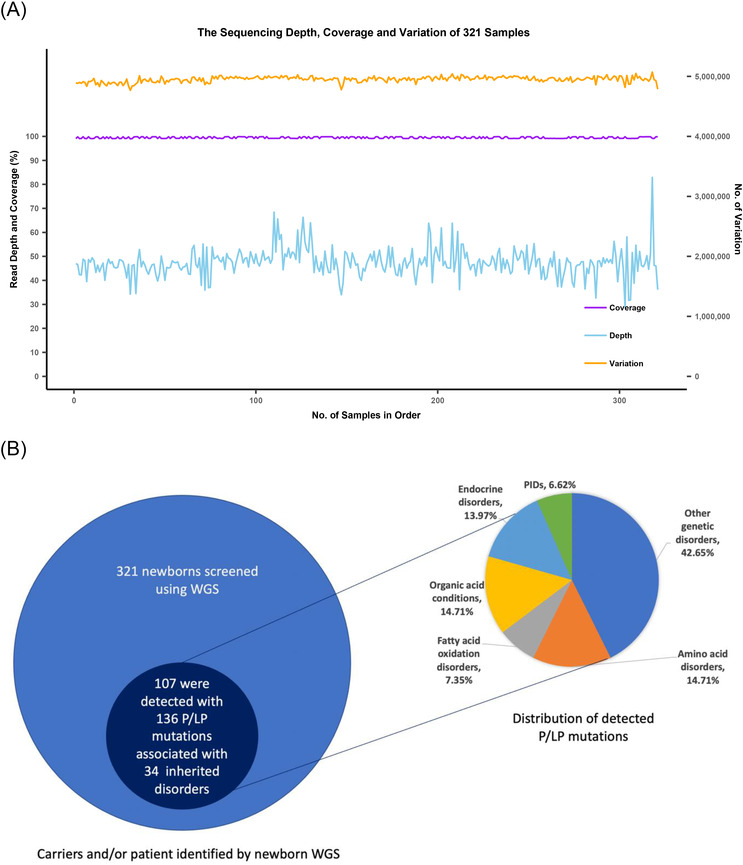
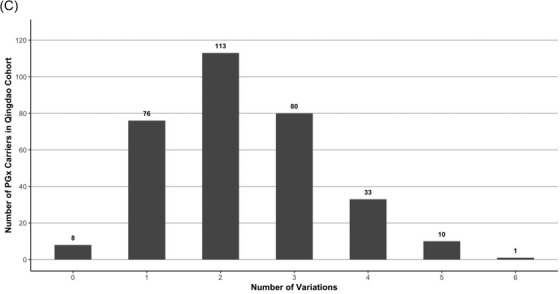
FIGURE 1.
Overview of the results of newborn WGS in the Qingdao cohort (n = 321). (A) The sequencing quality of WGS results in the Qingdao cohort (n = 321). (B) Overview of carriers and/or patient identified by WGS in the Qingdao cohort (n = 321). A total of 107 carriers and/ or patients associated with 34 inherited disorders were identified in the 321 newborns. Of the 136 P/LP mutations, 42.65% corresponded to genetic disorders, 6.62% were associated with PIDs and 50.74% were related to metabolic disorders, including organic acid conditions (14.71%), amino acid disorders (14.71%), fatty acid oxidation disorders (7.35%) and endocrine disorders (13.97%). Hearing loss, MMA, CH and PKU were the most common diseases with carriers, while GJB2 (28/321, 8.72%), MMACHC (11/321, 3.43%), DUOX2 (10/321, 3.12%), PAH (8/321, 2.49%) and SLC26A4 (8/321, 2.49%) were the top five genes with the highest carrier frequencies of P/LP mutations. For the 164 PIDs recognized by the IUIS, 9 heterozygous P/LP variants in 6 genes, corresponding to 6 diseases, were identified in 9 of the 321 newborn children (2.80%), all in a heterozygous state. Of these, there are two genes (ADA and JAK3) for which we found two carriers. (C) Distribution of actionable PGx variants identified by WGS in the Qingdao cohort (n = 321). A clinical management strategy can be adopted for every carrier according to the DPWG guidelines. Among 321 newborns, 313 newborns (97.51%) in the Qingdao cohort carried at least one clinically relevant variant, while 193 newborns (60.12%) harboured two and/or three variants. From the perspective of the five crucial pharmacogenes, the CYP2D6 gene had the highest carrier frequency, where 266 out of the 321 infants (82.87%) harboured at least one actionable PGx variant. The gene CYP2C19 showed the second‐highest carrier rate, where 209 infants (65.11%) carried at least one clinically relevant variant. In addition, 133 and 122 infants carried actionable PGx variants at the gene UGT1A1 and NUDT15, respectively. No actionable variant was identified in the DPYD gene in the 321 children. Among the selected gene–drug pairs, irinotecan, azathioprine, mercaptopurine and tioguanine were prescription drugs to paediatric patients, while codeine and clopidogrel are restricted for use of children under 18 years old. Our findings suggest that participants may obtain benefit from PGx profiling already in early childhood