Abstract
Background
Before August 2021, the only regimen recommended by the World Health Organization (WHO) to treat pediatric drug-susceptible tuberculous meningitis was a 12-month regimen consisting of isoniazid, rifampicin, ethambutol, and pyrazinamide (2HRZE/10HR). The comparative effectiveness of shorter regimens is unknown.
Methods
To inform a WHO guideline update, we undertook a systematic review and meta-analysis to evaluate outcomes from regimens of 6- to less than 12-months’ duration that included, at a minimum, isoniazid, rifampicin, and pyrazinamide. We included studies that applied rigorous diagnostic criteria and reported outcomes for ≥10 children or adolescents. Using generalized linear mixed models, we estimated the random effects pooled proportions of patients with key outcomes.
Results
Of 7 included studies, none compared regimens head-to-head. Three studies (724 patients) used a 6-month intensive regimen, which includes isoniazid and rifampicin at higher doses, pyrazinamide, and ethionamide instead of ethambutol (6HRZEto). Outcomes for this versus the 12-month regimen (282 patients, 3 studies) were, respectively, as follows: death, 5.5% (95% confidence interval [CI], 2.1%–13.4%) vs 23.9% (95% CI, 17.5%–31.7%); treatment success (survival with or without sequelae), 94.6% (95% CI, 73.9%–99.1%) vs 75.4% (95% CI, 68.7%–81.1%); and neurological sequelae among survivors, 66.0% (95% CI, 55.3%–75.3%) vs 36.3% (95% CI, 30.1%–43.0%). Relapse did not occur among 148 patients followed-up for 2 years after completing the 6-month intensive regimen.
Conclusions
Our findings are limited by the small number of studies and substantial potential for confounding. Nonetheless, the 6HRZEto regimen was associated with high treatment success and is now recommended by WHO as an alternative to the 12-month regimen.
Keywords: neurological sequelae, treatment outcomes, tuberculosis, World Health Organization guidelines
Compared with a 12-month regimen consisting of isoniazid, rifampicin, pyrazinamide, and ethambutol (2HRZE/10HR), an intensive 6-month regimen—which replaces ethambutol with ethionamide (6HRZEto)—is associated with lower mortality but more frequent neurological sequelae among survivors of pediatric tuberculous meningitis.
Tuberculous (TB) meningitis is associated with a 19.3% risk of death and 36.7% risk of neurological sequelae among surviving children and adolescents [1]. Despite poor outcomes, evidence to support current treatment approaches is limited. Before August 2021, the only regimen recommended by the World Health Organization (WHO) to treat pediatric drug-susceptible TB meningitis was a 12-month regimen, consisting of daily isoniazid, rifampicin, ethambutol, and pyrazinamide at standard doses for 2 months, followed by daily isoniazid and rifampicin for 10 months (2HRZE/10HR) [2, 3]. Regimens of less than 12 months’ duration, which may facilitate treatment completion and reduce burdens on patients and healthcare systems, are routinely used in some settings based on expert opinion [4, 5], but their effectiveness compared to the 12-month regimen is unknown. A 2014 systematic review and meta-analysis of pediatric TB meningitis outcomes observed no associations between treatment duration and outcomes [1]. However, the meta-analysis included studies of outdated regimens, and it did not control for confounding [1]. Although a randomized controlled trial is underway to compare a 6-month regimen of isoniazid, rifampicin, pyrazinamide, and levofloxacin against the WHO recommended 12-month regimen, results are not expected for a few more years [6].
To inform an update of WHO guidance on TB meningitis treatment in children (ages 0–9 years) and adolescents (ages 10–19 years), we undertook a systematic review and meta-analysis of outcomes from regimens that included, at a minimum, isoniazid, rifampicin, and pyrazinamide, and were given for 6 to less than 12 months. We sought to determine whether the risks of death, loss to follow-up, treatment success, and neurological sequelae were different when a regimen of 6 to less than 12 months’ duration was used, compared to the 12-month regimen.
METHODS
The protocol for this systematic review was registered on PROSPERO on April 12, 2021 (CRD 42021243817).
Search Strategy
An academic librarian (G.G.) adapted the search strategy used in a previous systematic review of pediatric TB meningitis outcomes [1] (Supplementary Text S1). Search terms included those related to the treatment of TB meningitis, children/adolescents, and outcomes. We ran the search on February 24, 2021, in PubMed, EMBASE Classic + EMBASE (Ovid), Web of Science Core Collection (Web of Science), BIOSIS Citation Index (Web of Science), Cochrane CENTRAL (Trials) (Cochrane Library), and LILACS (Global Index Medicus). We also considered unpublished studies/datasets proposed by the WHO Secretariat.
Screening and Data Abstraction
We screened studies in English, Spanish, French, Italian, Portuguese, Romanian, German, Chinese, Russian, and Ukrainian. Supplementary Text S2 provides detailed inclusion and exclusion criteria. In brief, we included studies that applied rigorous diagnostic criteria (see Supplementary Text S2) and reported our outcomes of interest for at least 10 children or adolescents treated with eligible regimens. Regimens had to include isoniazid, rifampicin, and pyrazinamide, with a treatment duration of 6 to less than 12 months. We excluded studies restricted to specific subsets of patients, such as those requiring shunt placement.
Title and abstract screening, full-text screening, and data abstraction were conducted independently by 2 investigators; disagreements were resolved by consensus or arbitration by a third investigator. We contacted study authors to ask for outcomes stratified by disease stage at diagnosis and other clarifying information, but we did not receive further data from the published studies of the 12-month regimen.
Risk of Bias Assessment
Given the lack of validated criteria for assessing the risk of bias in TB treatment studies, recent systematic reviews that have informed WHO TB guidelines have evaluated risk of bias in studies using tailored approaches informed by existing tools [7–9]. Similarly, we developed a checklist to assess 5 key sources of bias: (1) participant selection and loss to follow-up; (2) diagnostic uncertainty; (3) treatment allocation, including confounding by indication, and adherence; (4) assessment and reporting of treatment outcomes; and (5) potential confounding by age, human immunodeficiency virus (HIV) status, disease stage at diagnosis, and Mycobacterium tuberculosis resistance pattern (Supplementary Table S1). Each domain consisted of several subdomains relevant to pediatric TB meningitis. The risk of bias arising from each subdomain was judged to be low, high, or uncertain. Two investigators (1) independently applied the risk of bias tool to all included studies, (2) resolved discrepancies by discussion or consultation with a third investigator, and (3) summarized findings in a color-coded table. We did not generate an overall impression of bias for each study.
Statistical Analysis
Using generalized linear mixed models, we estimated the random effects pooled proportion and 95% confidence interval (CI) of patients with each of 5 outcomes: (1) death; (2) loss to follow-up; (3) treatment success, defined as the number of patients who completed treatment and were alive, with or without neurological sequelae; (4) neurological sequelae, defined as the number of survivors who completed treatment (ie, were not lost to follow-up) and developed neurological sequelae of any severity; and (5) survival without neurological sequelae. For neurological sequelae, the denominator was the number of patients who survived and completed treatment. For all other outcomes, the denominator was the total number of patients starting treatment.
We assessed between-study heterogeneity through visual inspection of forest plots. Because of the noncomparative nature of the studies, we did not pool measures of relative effect. Insufficient data precluded subgroup analyses (Supplementary Table S2). Analyses were performed using the meta package in R version 3.6.3 [10, 11].
Patient Consent Statement
As a systematic review and meta-analysis of aggregate-level data, this study did not require institutional board approval or patient consent.
RESULTS
From 1820 unique citations, we identified 130 studies for full-text screening. We also screened the 19 studies included in a prior systematic review [1] and 2 unpublished datasets identified by the WHO Secretariat [12]. One of those unpublished datasets has since been published [12]. Seven studies met inclusion criteria [12–17] (Supplementary Figure S1). Supplementary Table S3 reports reasons why individual studies were excluded after full-text review.
Characteristics of Included Studies
Table 1 summarizes the characteristics of the included studies, all of which were cohort studies, and none of which performed head-to-head comparisons of the regimens of interest [12–17]. Of 837 patients who received regimens of less than 12 months’ duration, 100 received an 8-month regimen in Vietnam (2HRZES/1HRZE/5HRE) [13], and 737 were treated at a single referral center in South Africa [14, 15]. Among the 737 patients in South Africa, 724 received the 6-month intensive (“Cape Town”) regimen, which consisted of daily isoniazid, rifampicin, pyrazinamide, and ethionamide throughout treatment (6HRZEto). As detailed in Table 1, dosing of isoniazid and rifampicin was higher compared to the 12-month regimen. For an additional 13 patients with HIV, the intensive regimen was extended to 9 months (9HRZEto) [14]. Patients whose regimens were extended beyond 6 months for other reasons, including poor adherence, tuberculomas, and isoniazid monoresistance, were excluded. A total of 282 patients received the 12-month regimen. Two studies of the 12-month regimen were conducted in India [16, 17], while data for the third study that included the 12-month regimen were collected in various centers in Europe through the Pediatric Tuberculosis Network European Trials Group (ptbnet) [12].
Table 1.
Main Characteristics of Studies Reporting on the Effectiveness of Regimens to Treat Drug-Susceptible Tuberculous Meningitis in Children and Adolescents
| Study Reference | Study Design | Location | Years of Enrollment | Duration of Follow-up | Number of Patients | Treatment Regimen | Patients Receiving Steroids | Patient Characteristics | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Female [n (%)] | HIV Status | Disease Stage (n per Stage) b |
||||||||
| Intervention Regimensa | |||||||||||
| Bang et al [13] | PC | Ho Chi Minh City, Vietnam | 2009–2011 | 8 months | 100 | 2HRZES/1HRZE/5HRE: H 5 mg/kg, R 10 mg/kg, Z 25 mg/kg, E 15 mg/kg, S 15 mg/kg | All | Median: 2.7 years (IQR 0.9–6.9 years) Range: 0.2–15 years |
44 (44.0) | 4/96 HIV-positive | 59 stage 1; 23 stage 2; 18 stage 3 |
| van Toorn et al [14] | PC | Western Cape, South Africa | 2006–2009 | ≥2 years after treatment completion | 135 | 6HRZEto: H 20 mg/kg, R 20 mg/kg, Z 40 mg/kg, Eto 20 mg/kg | All | Median: 2.9 years (IQR 1.5–7 years) Range: 0.2–14 years |
72 (53.3) | 6/135 HIV-positive | 16 stage 1; 68 stage 2; 51 stage 3 |
| 13 | 9HRZEto: H 20 mg/kg, R 20 mg/kg, Z 40 mg/kg, Eto 20 mg/kg | All | Median: 5.5 years (IQR 2.2–7.4 years) Range: 0.8–11 years |
3 (23.1) | All HIV-positive | 2 stage 1; 8 stage 2; 3 stage 3 |
|||||
| van Well et al [15] | RC | Western Cape, South Africa | 1985–2005 | 6 months | 554 | 6HRZEto: H 20 mg/kg, R 20 mg/kg, Z 40 mg/kg, Eto 20 mg/kg | 63% | Median: 2.3 years (IQR 1.3–4.2 years) Range: 0.2–15 years |
263 (47.5) | 8/213 HIV-positive | 14 stage 1; 318 stage 2; 222 stage 3 |
| Solomons et al (unpublished) | RC | Western Cape, South Africa | 2011–2014 | 6 months | 35 | 6HRZEto: H 20 mg/kg, R 20 mg/kg, Z 40 mg/kg, Eto 20 mg/kg | All | Median: 2.5 years (IQR 1.3–3.7 years) Range: 0.4–6.8 years |
16 (45.7) | 3/35 HIV-positive | 6 stage 1; 15 stage 2; 14 stage 3 |
| Comparator (WHO) Regimena | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dhawan et al [16] | PC | India | 2010–2013 | 12 months after hospital discharge | 130 | 2HRZE/10HR: H 10 mg/kg, R 15–20 mg/kg, Z 35–40 mg/kg, E 20–25 mg/kg | All | NR | 50 (38.5) | All HIV-negative | 26 stage 1; 56 stage 2; 48 stage 3 |
| Gupta et al [17] | PC | Delhi, India | 2012–2014 | 12 months | 138 | 2HRZE/10HR: H 10–20 mg/kg, R 10–20 mg/kg, Z 30–35 mg/kg, E 15–20 mg/kg | NR | <18 years | NR | NRc | NR |
| Thee et al [12] | RC | Europe (multiple countries) | 2009–2016 | 12 months | 14 | 2HRZE/10HR: H 9.8 (5.8–12.1) mg/kg, R 11.8 (10.1–14.5) mg/kg, Z 28.8 (25.0–31.3) mg/kg, E 19.2 (17.0–20.1) mg/kgd | All | Median: 3.3 years Range: 1–16 years |
5 (35.7) | All HIV-negative | 2 stage 1; 11 stage 2; 1 stage 3 |
Abbreviations: E, ethambutol; Eto, ethionamide; H, isoniazid; HIV, human immunodeficiency virus; IQR, interquartile range; NR, not reported; PC, prospective cohort; RC, retrospective cohort; R, rifampin; SD, standard deviation; S, streptomycin; WHO, World Health Organization; Z, pyrazinamide.
Regimens of 6 to <12 months’ duration were classified as “intervention,” whereas 12-month regimens were classified as “comparator” (WHO).
Disease stage was defined in accordance with the British Medical Research Council scale in all studies; however, children younger than 5 years in Bang et al [13] were staged as per the Blantyre Coma Scale.
The study included both adults and children; 12 HIV-positive individuals were included in the entire cohort but their age distribution was not reported.
Reported doses are the median (25th and 75th percentile) doses given for each drug.
Among cohorts that received regimens of less than 12 months’ duration, the median age of patients ranged from 2.3 to 2.9 years. The median age of the ptbnet patients who received the 12-month regimen was 3.3 years; summary age data were not reported for the Indian cohorts. Children with HIV comprised 2.5% of patients who received the 6-month intensive regimen. Two of 3 cohorts that received regimens of 12 months’ duration did not include patients with HIV [12, 16]. The ages of the 12 patients with HIV included in the third study of 12-month regimen, which combined adults and children, were not reported [17].
Patients who received the 6-month intensive or 12-month regimens were staged at diagnosis using the original or modified British Medical Research Council (MRC) staging system [12, 14–16]. One study of the 12-month regimen did not report stage [17]. The Vietnam study used the MRC classification for patients 5 years or older and the Blantyre coma scale for patients younger than 5 years [13]. Of the 724 patients who received the 6-month intensive regimen, 36 (5.0%) presented in stage 1, 401 (55.4%) presented in stage 2, and 287 (39.6%) presented in stage 3. Of the 282 patients who received the 12-month regimen, 28 (9.9%) presented in stage 1, 67 (23.8%) presented in stage 2, and 49 (17.4%) presented in stage 3; stage was not reported for 138 (48.9%) patients. Fifty-nine percent of the patients in the Vietnamese cohort were diagnosed in stage 1 (Table 1). Supplementary Tables S4 and S5 summarize additional patient characteristics.
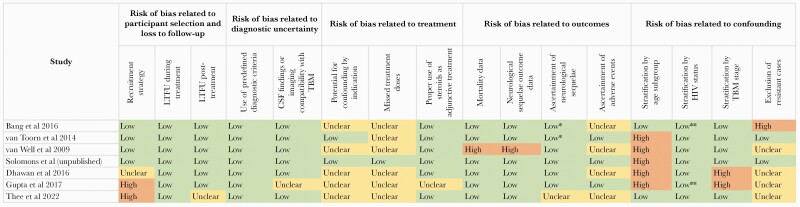
Risk of Bias in Included Studies
Figure 1 presents the results of the risk of bias assessment. All studies of regimens of 6 to less than 12 months’ duration had low risks of selection bias because they included all consecutive patients meeting eligibility criteria and had few losses to follow-up [13–15]. However, studies of the 12-month regimen had high or unclear risk of selection bias because they did not enroll consecutive patients [12, 17] or did not provide information on sampling approaches [16]. Risk of bias related to diagnostic uncertainty was uncommon because all studies applied prespecified diagnostic criteria, which, in all but 1 study, included cerebrospinal fluid (CSF) assays and/or central nervous system imaging.
Figure 1.
Summary results of risk of bias assessment of 7 reporting on the effectiveness of regimens to treat drug-susceptible tuberculous meningitis in children and adolescents. *, Neurological sequelae were ascertained through both clinical exam and reports from patients/caregivers. **, Although stratum-specific data were not available, this was considered of limited concern given the very small number of human immunodeficiency virus (HIV)-positive patients included in the cohort. CSF, cerebrospinal fluid; LTFU, loss to follow-up; TBM, tuberculous meningitis.
Risk of bias related to treatment allocation was unclear for 5 studies because reasons for regimen choices were unreported [12, 13, 15–17]. Risk of bias related to treatment allocation was low for 2 cohorts that received the 6-month intensive regimen because the reasons for extending therapy beyond 6 months were explained, and we were able to exclude those patients from the meta-analysis [14]. Treatment adherence, which may have impacted effectiveness, was reported in only 1 unpublished dataset from South Africa. Steroid use was inconsistent in the largest cohort that received the 6-month intensive regimen [15] and was not reported in one cohort that received the 12-month regimen [17].
Risk of bias related to treatment outcome reporting was high for one study of the 6-month intensive regimen because over 10% of patients were missing outcomes [15]. For the unpublished study of the 12-month regimen, there was uncertain risk of bias related to ascertainment of neurological sequelae, which were not standardized across the 9 study sites [12]. Risk of bias related to adverse events was unclear in 4 studies due to a lack of data [12, 13, 15, 16].
Confounding was the domain with the highest concern for bias. Outcomes were not reported for age subgroups, thus hindering assessment of age-related differences. Two studies of the 12-month regimen did not disaggregate outcomes by disease stage [16, 17], the best-known predictor of outcome. Finally, most studies provided limited details regarding efforts to exclude drug-resistant cases. No study reported the resistance profiles of presumed source cases. In 3 cohorts that received the 6-month intensive regimen, drug susceptibility testing was conducted in fewer than a third of patients [14, 15]. The remaining studies did not report susceptibility testing [12, 13, 16, 17].
Treatment Outcomes
All studies reported mortality, treatment success, and neurological sequelae among survivors. All but 1 study reported loss to follow-up [12–16]. Only 1 study from South Africa reported relapse [14].
All studies of the 6-month intensive regimen stratified death and neurological sequelae by stage at diagnosis. No studies stratified outcomes by age, sex, nutritional status, isoniazid susceptibility pattern, microbiological confirmation, or complications (Supplementary Table S2).
One cohort of 13 patients with HIV who received a 9-month intensive regimen in South Africa and another cohort of 100 patients who received an 8-month regimen in Vietnam were excluded from meta-analysis [13, 14]. The proportions of patients who received the 9-month intensive regimen that died (1) were lost to follow-up, (2) were successfully treated, and (3) had neurological sequelae fell within the 95% CIs of those proportions in the cohorts that received the 6-month intensive regimen. Likewise, outcomes for patients who received the 8-month regimen were similar to those of the cohorts that received the 12-month regimen (Tables 2 and 3).
Table 2.
Proportions of Death, Loss to Follow-up, and Treatment Success Across Studies Reporting on the Effectiveness of Regimens to Treat Drug-Susceptible Tuberculous Meningitis in Children and Adolescents
| Study | Regimen | Number Started on Treatment | Number (%) Lost to Follow-up |
Number (%) of Deaths by End of Treatment |
Number (%) of Patients With Treatment Successa | ||||
|---|---|---|---|---|---|---|---|---|---|
| Bang et al [13] | 2HRZES/1HRZE/5HRE | 100 | Stage 1: 59 Stage 2: 23 Stage 3: 18 |
4 (4.0) All patients lost to follow-up were in stage 1 |
15 (15.0) 14/15 deaths occurred <45 days of diagnosis |
Stage 1: 1 (1.7) Stage 2: 4 (17.4) Stage 3: 10 (55.6) |
81 (81.0) | Stage 1: 91.5% Stage 2: 82.6% Stage 3: 44.4% |
|
| van Toorn et al [14] | 6HRZEto | 135 | Stage 1: 16 Stage 2: 68 Stage 3: 51 |
0 | 6 (4.4) All deaths occurred <8 days of treatment initiation in patients who were moribund at presentation |
Stage 1: 0 (0) Stage 2: 0 (0) Stage 3: 6 (11.8) |
129 (95.6) | Stage 1: 100% Stage 2: 100% Stage 3: 82.5% |
|
| 9HRZEto | 13 (all HIV-pos) | Stage 1: 2 Stage 2: 8 Stage 3: 3 |
0 | 1 (7.7) Patient in stage 3 |
12 (92.3) | Stage 1:100% Stage 2: 100% Stage 3: 33.3% |
|||
| van Well et al [15] | 6HRZEto | 554 | Stage 1: 14 Stage 2: 318 Stage 3: 222 |
66b (11.9) | Stage 1: 1 (7.1) Stage 2: 17 (5.3) Stage 3: 48 (21.6) |
53 (9.6) | Stage 1: 0 (0) Stage 2: 11 (3.5) Stage 3: 42 (18.9) |
435 (78.5) | Stage 1: 92.9% Stage 2: 91.2% Stage 3: 59.5% |
| Solomons et al (unpublished) | 6HRZEto | 35 | Stage 1: 6 Stage 2: 15 Stage 3: 14 |
0 | 0 | 35 (100) | Stage 1: 100% Stage 2: 100% Stage 3: 100% |
||
| Dhawan et al [16] |
2HRZE/10HR | 130 | Stage 1: 26 Stage 2: 56 Stage 3: 48 |
0 | 39 (30.0) -38/39 deaths occurred during hospitalization (early phase of treatment), and 1/39 occurred 2 months after hospital discharge -Stage-specific outcomes not reported, but stage 3 was strongest risk factor for mortality |
91 (70.0) | Stage not reported | ||
| Gupta et al [17] |
2HRZE/10HR | 138 | Stage not reported | Not reportedb | 29 (21.0) No further details provided |
109 (79.0) | Stage not reported | ||
| Thee et al [12] | 2HRZE/10HR | 14 | Stage 1: 2 Stage 2: 11 Stage 3: 1 |
1 (7.1) Patient lost to follow-up was in stage 2 |
1 (7.1) Patient in stage 3 |
12 (85.7) | Stage 1: 100% Stage 2: 90.9% Stage 3: 0 |
||
Abbreviations: d, day; E, ethambutol; Eto, ethionamide; H, isoniazid; HIV, human immunodeficiency virus; pos, positive; R, rifampicin; Z, pyrazinamide.
Treatment success was defined as the number of patients who were still alive, with or without sequelae, and had completed treatment.
This includes 53 patients who were likely alive and had completed treatment, but whose outcome was not recorded and whose neurological status was not assessed.
Table 3.
Neurological Sequelae Among Survivors Across Studies Reporting on the Effectiveness of Regimens to Treat Drug-Susceptible Tuberculous Meningitis in Children and Adolescents
| Study | Regimen | Definition of Neurological Outcomes |
Number Alive at End of Treatment |
Number (%) With Neurological Sequelae |
||
|---|---|---|---|---|---|---|
| Bang et al [13] | 2HRZES/1HRZE/5HRE | Severe or intermediate disability (not further specified) | 81 | Stage 1: 54 Stage 2: 19 Stage 3: 8 |
27 (33.3) Moderate: 21 (25.9) Severe: 6 (7.4) |
Stage 1: 11 (20.4) Stage 2: 10 (52.6) Stage 3: 6 (75.0) |
| van Toorn et al [14] | 6HRZEto | Mild sequelae: • mild intellectual impairment, • hemiparesis • impaired vision and/or hearing Severe sequelae: • severe intellectual impairment, • quadriparesis, • blindness and/or deafness. |
129 | Stage 1: 16 Stage 2: 68 Stage 3: 45 |
71 (55.0) Mild: 49 (38.0) Severe: 22 (17.1) |
Stage 1: 1 (6.3) Stage 2: 27 (39.7) Stage 3: 43 (95.6) |
| 9HRZEto | 12 | Stage 1: 2 Stage 2: 8 Stage 3: 2 |
6 (50.0) Mild: 3 (25.0) Severe: 3 (25.0) |
Stage 1: 1 (50.0) Stage 2: 3 (37.5) Stage 3: 2 (100) |
||
| van Well et al [15] | 6HRZEto | 435 | Stage 1: 13 Stage 2: 290 Stage 3: 132 |
294 (66.7) Mild: 217 (49.9) Severe: 77 (17.7) |
Stage 1: 2 (15.4) Stage 2: 182 (62.8) Stage 3: 110 (83.3) |
|
| Solomons et al (unpublished) | 6HRZEto | 35 |
Stage 1: 6 Stage 2: 15 Stage 3: 14 |
28 (80.0) | Stage 1: 3 (50.0) Stage 2: 13 (86.7) Stage 3: 12 (85.7) |
|
| Dhawan et al [16] | 2HRZE/10HR | Mild/moderate/severe disability, coma, or vegetative state |
91 | Not disaggregated by stage, but stage 3 was the strongest risk factor for poor neurological outcome | 29 (31.9) Mild: 17 (18.1) Moderate: 5 (5.5) Severe: 4 (4.4) Coma or vegetative state: 3 (3.3) |
|
| Gupta et al [17] | 2HRZE/10HR | Altered sensorium, cranial nerve palsy, extrapyramidal movements, focal neurological deficit, mental retardation, optic atrophy, and/or tone abnormalities |
109 | Not disaggregated by stage | 42 (38.5) No further details provided |
|
| Thee et al [12] | 2HRZE/10HR | Coma, paresis, spasticity, cranial nerve palsy, seizures, hydrocephalus, hypothalamic or pituitary dysfunction, developmental delay, impairment of speech, hearing, or vision | 12 | Stage 1: 2 Stage 2: 10 Stage 3: 0 |
6 (50.0) | Stage 1: 0 Stage 2: 6 (60.0) Stage 3: 0 |
Abbreviations: E, ethambutol; Eto, ethionamide; H, isoniazid; NR, not reported; R, rifampicin; S, streptomycin.
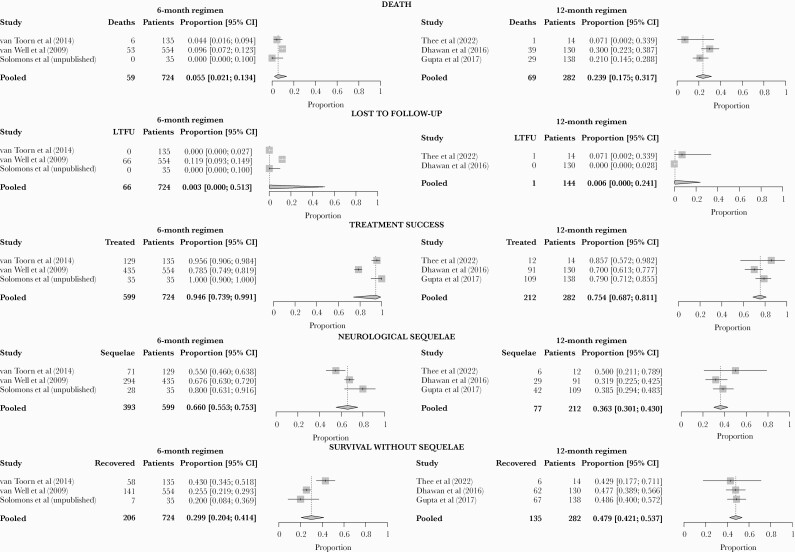
Compared to the 12-month regimen, the 6-month intensive regimen was associated with a lower proportion of death, similar proportion of loss to follow-up, and higher proportions of treatment success and neurological sequelae. Death ranged from 0% to 9.6%, with a pooled proportion of 5.5% (95% CI, 2.1%–13.4%) for the 6-month intensive regimen, and from 7.1% to 30.0%, with a pooled proportion of 23.9% (95% CI, 17.5%–31.7%) for the 12-month regimen (Table 2 and Figure 2). Heterogeneity was limited for the 6-month intensive regimen but substantial for the 12-month regimen.
Figure 2.
Forest plot of pooled proportions of death, loss to follow-up, treatment success, neurological sequelae, and survival without sequelae across 3 studies of 6-month intensive regimen (6HRZEto) and 3 studies of 12-month standard regimen (2HRZE/10HR). One study of the 12-month regimen (Gupta et al [17]) was excluded from analysis of loss to follow-up because it only included patients with complete follow-up period. In van Well et al [15], 53 of 66 patients who were counted as lost to follow-up were likely alive and had completed treatment, but their outcome was not recorded, and their neurological status could not be assessed. CI, confidence interval; LTFU, loss to follow-up.
No patients were lost to follow-up in 2 of 3 cohorts that received the 6-month intensive regimen [14]; 11.9% of patients were lost to follow-up in the third cohort [15]. Two of three studies of the 12-month regimen reported loss to follow-up [12, 16], which occurred in 0% and 7.1% of patients (Table 2 and Figure 2). The pooled proportions of loss to follow-up were 0.3% (95% CI, .0%–51.3%) and 0.6% (95% CI, .0%–24.1%) for the 6-month intensive and 12-month regimens, respectively.
Treatment success ranged from 78.5% to 100.0%, with a pooled proportion of 94.6% (95% CI, 73.9–99.1) for the 6-month intensive regimen, and from 70% to 85.7%, with a pooled proportion of 75.4% (95% CI, 68.7–81.1) for the 12-month regimen (Table 2 and Figure 2). Heterogeneity was moderate for the 6-month intensive regimen but minimal for the 12-month regimen.
Neurological sequelae were defined and assessed differently between study sites (Table 3). Fifty to 66.7% of survivors successfully treated with the 6-month intensive regimen had neurological sequelae, mostly mild. Among survivors successfully treated with the 12-month regimen, 31.9%–50.0% had neurological sequelae. In one study from India, 17 of 29 (58.6%) patients with sequelae had mild sequelae [16]. The other studies of the 12-month regimen did not report severity. The pooled proportions of survivors who completed treatment and had neurological sequelae were 66.0% (95% CI, 55.3%–75.3%) and 36.3% (95% CI, 30.1%–43.0%) for the 6-month intensive regimen and the 12-month regimen, respectively (Figure 2). Heterogeneity was substantial for both regimens.
The proportion of patients who completed treatment and survived without sequelae was 20.0%–43.0%, with a pooled proportion of 29.9% (95% CI, 20.4%–41.4%) for the 6-month intensive regimen; among those who received the 12-month regimen, the proportions ranged from 42.9% to 48.6% (Supplementary Table S6), with a pooled proportion of 47.9% (95% CI, 42.1%–53.7%). Heterogeneity was moderate for the 6-month intensive regimen and limited for the 12-month regimens.
Only 1 study reported relapse. Among 148 patients who received the 6-month intensive regimen, none relapsed within 2 years posttreatment [14].
Adverse Events
Only 3 studies reported drug-related adverse events [13, 14, 16]. Hepatotoxicity was the most frequently reported event (Supplementary Table S7). In one study of the 6-month intensive regimen, hepatotoxicity resolved after a change to a less hepatotoxic regimen and subsequent stepwise restarting of the original regimen [14]. In Vietnam, 2 patients developed drug-induced hepatotoxicity (defined per the WHO definition) and recovered after discontinuation of pyrazinamide [13, 18]. The remaining study did not explain the approach to managing hepatotoxicity [16].
DISCUSSION
In this systematic review and meta-analysis, we found that, compared to the 12-month regimen, the 6-month intensive regimen was associated with lower mortality, higher treatment success, and more frequent neurological sequelae among survivors. Loss to follow-up was similar between the regimens, but data on this outcome were incomplete. The cohort in Vietnam had similar outcomes as those that received the 12-month regimen [13]. Data were insufficient to compare adverse events. A key concern with shorter TB regimens is relapse; however, in one study of the 6-month intensive regimen, none of the 148 patients who were monitored for 2 years posttreatment relapsed [14]. Moreover, across studies and regimens, almost all deaths occurred in the early treatment phases. Taken together, these observations suggest that treatment success may depend more on early effective treatment than on regimen duration beyond 6 months.
These findings should be interpreted with caution. As highlighted in our risk of bias assessment, there was high potential for confounding by indication, disease stage at diagnosis, treatment adherence, and other patient characteristics. A range of antimicrobial doses were prescribed to patients who received the 12-month regimen, including within studies (Table 1). However, because outcomes were not disaggregated by dose, we could not evaluate for associations between these 2 variables. All data for the 6-month intensive regimen came from a single referral center in South Africa [14, 15], whereas most patients who received the 12-month regimen were treated in India [16, 17]. These distinct settings may lead to additional sources of confounding, including the following: time to diagnosis and treatment; the non-antimicrobial components of TB meningitis therapy, such as hydrocephalus management and steroid treatment; and perhaps even genetic differences in anti-TB drug metabolism [19, 20]. Finally, the lack of standardization of the assessment and categorization of neurological sequelae may account for differences in this outcome across sites.
The available data were insufficient to directly compare the effectiveness of pediatric TB meningitis regimens. Nevertheless, treatment success among patients who received the 6-month intensive regimen was 95%. The higher doses of isoniazid and rifampicin used in this regimen, together with the greater CSF penetration of ethionamide compared with ethambutol, may contribute to the effectiveness of the 6-month intensive regimen [21]. Rifampicin dosing may be particularly important for successful treatment [22]: data from adults with TB meningitis show an association between higher rifampicin concentrations and survival, and a small pediatric trial has shown improved neurocognitive function with doses of 30 mg/kg compared to the standard dose of 15 mg/kg [23, 24]. Nonpharmacological interventions also may have contributed to low mortality, but treatment success would not have been so high had the regimen been ineffective. Based on our findings, in August 2021, the WHO conditionally recommended the 6-month intensive regimen as an alternative to the 12-month regimen for treating TB meningitis in children and adolescents, for which a strong recommendation remains in place [3].
The current evidence base on pediatric TB meningitis treatment is limited. Despite an extensive search, we identified only 7 datasets meeting inclusion criteria. Few studies report outcomes from the regimens of interest, and many studies—particularly those reporting on the 12-month regimen—have design limitations. Children and adolescents with TB meningitis often are managed in hospitals at the start of treatment and then transferred to outpatient clinics to complete therapy; most hospital-based studies do not collect data after discharge and, thus, do not report end-of-treatment outcomes. Some studies reported aggregated results from multiple regimens, including the 12-month regimen. Another limitation is inconsistent reporting of patient characteristics and treatment outcomes across studies. Currently, 2 clinical trials of pediatric TB meningitis regimens are underway [6, 25]. Although these interventional studies are important, they are challenging to carry out and subject to their own limitations; for instance, stricter enrollment criteria lead to highly selective participant populations [26].
Future studies on pediatric TB meningitis must improve upon limitations in the current literature. Collaboration across sites is critical to pediatric TB meningitis research. In addition, the implementation of standardized methods for the conduct and reporting of TB meningitis studies, as proposed by a group of experts in the field, would improve the evidence base and facilitate future meta-analyses [27]. These proposed standards include the following: the reporting of explicit indications for which deviations from the standard regimen occurred; the reporting of susceptibility patterns of patients’ M tuberculosis strains, confirmed through testing, if available, or presumed based on the source case’s susceptibility pattern; the stratification of outcomes by disease stage at diagnosis; and the application of standardized evaluations of neurological outcomes, adverse events, loss to follow-up, and relapse [28].
Finally, future research must address not only antimicrobial regimen composition, doses, and duration but also identify strategies for earlier diagnosis and treatment of TB meningitis; optimal anti-inflammatory therapies; and ways to prevent and treat paradoxical reactions, which may have devastating consequences for the patient [29]. Unless these limitations to pediatric TB meningitis research are addressed, too many children and adolescents will continue to succumb to or suffer long-term disability from this disease.
CONCLUSIONS
This meta-analysis of pediatric TB meningitis regimens is limited by the small number of studies that met inclusion criteria and substantial potential for confounding. Nonetheless, the 6-month intensive regimen was associated with high treatment success and is now recommended by the WHO as an alternative to the 12-month regimen.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Tamara Kredo, Lawrence Mbuagbaw, and Kelly Dooley for their valuable feedback. We also thank Yana Sheremeta for screening articles in Russian and Ukrainian, and we thank Sansu Chiang for screening articles in Chinese.
Author contributions . G. S., A. B., F. A. K., and S. S. C. designed the study with critical feedback from S.V., K. V., A. B., and T. M. G. G. prepared the search strategy. G. S. and G. T. performed title/abstract screening, full-text screening, data extraction, and assessment of risk of bias. F. A. K. and S. S. C. were consulted to solve discrepant judgements and finalize decisions with respect to study inclusion. S. T., R. S., R. v. T., and J. D. provided unpublished data and/or additional details about published studies included in this review. G. S. performed the qualitative synthesis, whereas G. T. conducted the meta-analyses under the guidance of A. B. G. S., S. S. C. and F. A. K. prepared the first draft of the manuscript, which was subsequently revised by all authors until finalization.
Disclaimer. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Financial support. This work was funded by the Global Tuberculosis Programme, World Health Organization, Geneva, Switzerland.
Potential conflicts of interest . All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Giorgia Sulis, Department of Epidemiology, Biostatistics and Occupational Health, School of Population and Global Health, McGill University, Montreal, Canada; McGill International TB Centre, Montreal, Canada.
Gamuchirai Tavaziva, McGill International TB Centre, Montreal, Canada.
Genevieve Gore, Schulich Library, McGill University, Montreal, Canada.
Andrea Benedetti, Department of Epidemiology, Biostatistics and Occupational Health, School of Population and Global Health, McGill University, Montreal, Canada; McGill International TB Centre, Montreal, Canada.
Regan Solomons, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
Ronald van Toorn, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
Stephanie Thee, Charité-Universitätsmedizin Berlin, Berlin, Germany.
Jeremy Day, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom; Oxford University Clinical Research Unit, Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam.
Sabine Verkuijl, Global Tuberculosis Programme, World Health Organization, Geneva, Switzerland.
Annemieke Brands, Global Tuberculosis Programme, World Health Organization, Geneva, Switzerland.
Kerri Viney, Global Tuberculosis Programme, World Health Organization, Geneva, Switzerland.
Tiziana Masini, Global Tuberculosis Programme, World Health Organization, Geneva, Switzerland.
Faiz Ahmad Khan, McGill International TB Centre, Montreal, Canada.
Silvia S Chiang, Department of Pediatrics, Alpert Medical School of Brown University, Providence, Rhode Island, USA; Center for International Health Research, Rhode Island Hospital, Providence, Rhode Island, USA.
References
- 1. Chiang SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14:947–57. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO). Guidance for national tuberculosis programmes on the management of tuberculosis in children (2nd edition). Geneva, Switzerland: Global Tuberculosis Programme, World Health Organization (WHO). Available at: http://apps.who.int/iris/bitstream/handle/10665/112360/9789241548748_eng.pdf;jsessionid=0ACFB572CB1E7641DFE70AE9A7290797?sequence=1. Accessed 26 January 2022. [Google Scholar]
- 3. World Health Organization. Rapid Communication on Updated Guidance on the Management of Tuberculosis in Children and Adolescents. Geneva: World Health Organization; 2021. [Google Scholar]
- 4. Guidelines for the Management of Tuberculosis in Children. Pretoria, South Africa: Department of Health; 2013. [Google Scholar]
- 5. Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH.. Tuberculosis. Red Book 2021: Report of the Committee on Infectious Diseases. Itasca, IL: American Academy of Pediatrics; 2021: pp 786–814. [Google Scholar]
- 6. SURE: Short Intensive Treatment for Children with Tuberculous Meningitis. London, United Kingdom: MRC Clinical Trials Unit at University College London. Available at: https://www.isrctn.com/ISRCTN40829906. Accessed 28 March 2022. [Google Scholar]
- 7. Bastos ML, Lan Z, Menzies D.. An updated systematic review and meta-analysis for treatment of multidrug-resistant tuberculosis. Eur Respir J 2017; 49:1600803. [DOI] [PubMed] [Google Scholar]
- 8. Ahmad N, Ahuja SD, Akkerman OW, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018; 392:821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D.. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17:223–34. [DOI] [PubMed] [Google Scholar]
- 10. Balduzzi S, Rücker G, Schwarzer G.. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwarzer G. Meta: an R package for meta-analysis. Available at: https://cran.r-project.org/web/packages/meta/meta.pdf. Accessed 28 March 2022. [Google Scholar]
- 12. Thee S, Basu Roy R, Blázquez-Gamero D, et al. Treatment and outcome in children with tuberculous meningitis – a multi-centre Paediatric Tuberculosis Network European Trials Group study. Clin Infect Dis 2021:ciab982. doi: 10.1093/cid/ciab982. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13. Bang ND, Caws M, Truc TT, et al. Clinical presentations, diagnosis, mortality and prognostic markers of tuberculous meningitis in Vietnamese children: a prospective descriptive study. BMC Infect Dis 2016; 16:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Toorn R, Schaaf HS, Laubscher JA, van Elsland SL, Donald PR, Schoeman JF.. Short intensified treatment in children with drug-susceptible tuberculous meningitis. Pediatr Infect Dis J 2014; 33:248–52. [DOI] [PubMed] [Google Scholar]
- 15. van Well GT, Paes BF, Terwee CB, et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the Western Cape of South Africa. Pediatrics 2009; 123:e1–8. [DOI] [PubMed] [Google Scholar]
- 16. Dhawan SR, Gupta A, Singhi P, Sankhyan N, Malhi P, Khandelwal N.. Predictors of neurological outcome of tuberculous meningitis in childhood: a prospective cohort study from a developing country. J Child Neurol 2016; 31:1622–7. [DOI] [PubMed] [Google Scholar]
- 17. Gupta R, Kushwaha S, Thakur R, et al. Predictors of adverse outcome in patients of tuberculous meningitis in a multi-centric study from India. Indian J Tuberc 2017; 64:296–301. [DOI] [PubMed] [Google Scholar]
- 18. Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R.. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol 2008; 23:192–202. [DOI] [PubMed] [Google Scholar]
- 19. Abulfathi AA, Decloedt EH, Svensson EM, Diacon AH, Donald P, Reuter H.. Clinical pharmacokinetics and pharmacodynamics of rifampicin in human tuberculosis. Clin Pharmacokinet 2019; 58:1103–29. [DOI] [PubMed] [Google Scholar]
- 20. Matsumoto T, Ohno M, Azuma J.. Future of pharmacogenetics-based therapy for tuberculosis. Pharmacogenomics 2014; 15:601–7. [DOI] [PubMed] [Google Scholar]
- 21. Donald PR. The chemotherapy of tuberculous meningitis in children and adults. Tuberculosis (Edinb) 2010; 90:375–92. [DOI] [PubMed] [Google Scholar]
- 22. Panjasawatwong N, Wattanakul T, Hoglund RM, et al. Population pharmacokinetic properties of antituberculosis drugs in Vietnamese children with tuberculous meningitis. Antimicrob Agents Chemother 2020; 65(1):e00487-20. doi: 10.1128/AAC.00487-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Svensson EM, Dian S, Te Brake L, et al. Model-based meta-analysis of rifampicin exposure and mortality in Indonesian tuberculous meningitis trials. Clin Infect Dis 2020; 71:1817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Valvi C. OA24-763-21 High-dose rifampicin with or without levofloxacin for the treatment of paediatric tuberculous meningitis. 52nd Union World Conference on Lung Health. Virtual Event: The Union, 2021.
- 25. Optimizing treatment to improve TBM outcomes in children (TBM-KIDS). Available at: https://clinicaltrials.gov/ct2/show/NCT02958709. Accessed 28 March 2022.
- 26. Paradkar M, Devaleenal DB, Mvalo T, et al. Challenges in conducting trials for pediatric tuberculous meningitis: lessons from the field. Int J Tuberc Lung Dis 2019; 23:1082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marais BJ, Heemskerk AD, Marais SS, et al. Standardized methods for enhanced quality and comparability of tuberculous meningitis studies. Clin Infect Dis 2017; 64:501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davis AG, Nightingale S, Springer PE, et al. Neurocognitive and functional impairment in adult and paediatric tuberculous meningitis. Wellcome Open Res 2019; 4:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR.. Serial CT scanning in childhood tuberculous meningitis: prognostic features in 198 cases. J Child Neurol 1995; 10:320–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.