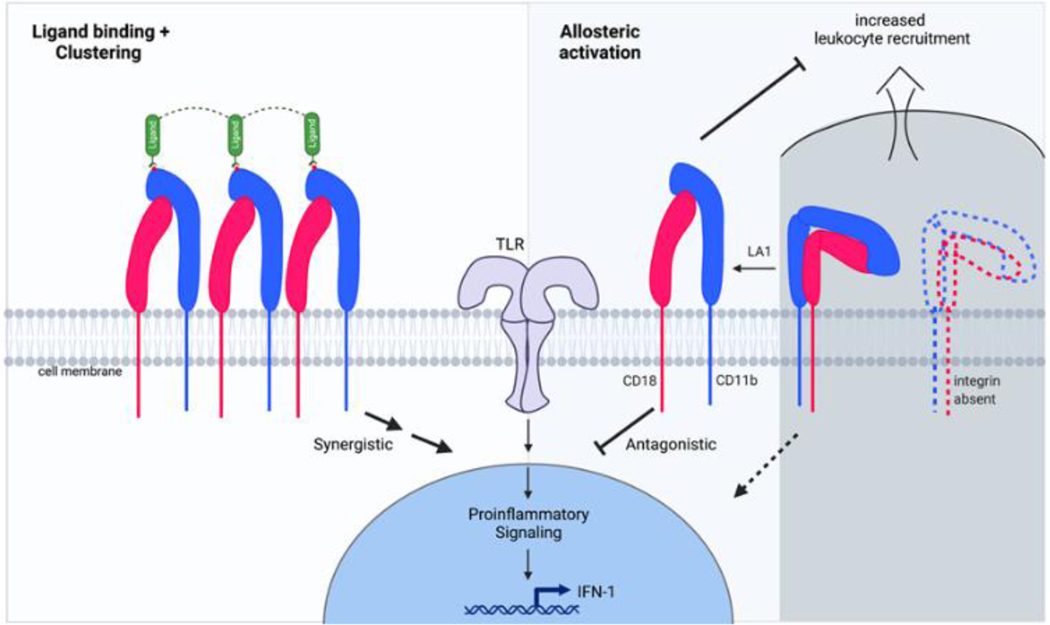
Figure 4. The many roles of CD11b in promoting or suppressing inflammatory pathways.
Ligand binding and clustering of CD11b/CD18 integrins on cell surface typically result in enhancing TLR-dependent pro-inflammatory signals and are involved in pathogen clearance, oxidative burst and other immune surveillance functions of the innate immune cells (left half). Absence of CD11b results in loss of this integrin on cell surface, that also promotes pro-inflammatory pathways, increased tissue recruitment of leukocytes, reduced ability to clear pathogens, yet increased expression of pro-inflammatory mediators, such as IL-6, IL-1βand TNFα, due to over-active TLR-dependent signaling pathways in CD11b−/− cells. Conversely, the presence of CD11b limits TLR-pathways and its allosteric activation, either pharmacologically or genetically, significantly dampens the exuberant TLR-dependent pro-inflammatory pathways in leukocytes, reduces leukocyte infiltration and tissue damage. Such activation is also able to rescue some of the functional deficits in cells carrying LN associated ITGAM SNPs, suggesting allosteric agonism of integrins as a novel therapeutic strategy.