Abstract
Cellular senescence is associated with normal development and wound healing, but has also been implicated in the pathogenesis of numerous aging-related diseases including osteoarthritis (OA). Treatment strategies for OA are being developed that target senescent cells and the paracrine and autocrine secretions of the senescence-associated secretory phenotype (SASP). The field of potential therapies continues to expand as new mechanistic targets of cell senescence and the SASP are identified. Ongoing pre-clinical and clinical studies of drugs targeting cellular senescence yield significant promise, but have yet to demonstrate long-term efficacy. Therapeutic targeting of senescence is challenged by the diverse phenotypes of senescent cells, which can vary depending on age, species, tissue source, and type of physiologic stressor. Accordingly, there remains considerable demand for more studies to further develop and assess senotherapeutics as disease-modifying treatments for OA.
Keywords: osteoarthritis, cell senescence, aging, cytokines, therapeutics
Introduction
Osteoarthritis (OA) is a chronic and debilitating condition that affects more than 30 million individuals in the United States [1]. The progression of OA is marked by the loss of articular cartilage, joint remodeling, and changes in the synovial membrane associated with inflammation. Current FDA-approved therapies for OA target symptoms of joint pain rather than the components of structural disease progression. Ongoing studies are investigating the potential for disease-modifying OA drugs (DMOADs). The most recent drugs to undergo phase II clinical trials include intra-articular sprifermin, a recombinant human fibroblast growth factor 18, intra-articular lorecivivint, a Wnt pathway modulator, and MIV-711, a selective cathepsin K inhibitor [2–5]. These trials demonstrate the potential for disease modification, although to date no drug has reached approval as a DMOAD. Given the correlation between cellular senescence and OA, drugs that target senescent cells, and their production of factors associated with the senescence-associated secretory phenotype (SASP) in joint tissues, have generated considerable interest for developing novel OA therapies.
Senescence is a natural cellular response to stressors including telomere dysfunction, DNA damage, and oncogene activation. It is characterized by arrested cellular proliferation and the induction of a distinctive secretory phenotype called the SASP [6,7]. Senescent cells contribute to tissue development during embryogenesis and to tissue repair during wound healing. These cells also suppress tumor formation by preventing the propagation of damaged cells. Although beneficial in certain contexts, the accumulation of excessive numbers of senescent cells (SnCs) has been implicated in the pathophysiology of many diseases associated with aging, including atherosclerosis, macular degeneration, and sarcopenia, as well as OA [8–12]. Rather than undergo apoptosis, senescent cells maintain upregulated pro-survival pathways such as ephrins, phosphatidylinositol 3-kinase (PI3K)-AKT, BCL-2 family proteins, p53-associated pathways, and FOXO4 [13–16]. These antiapoptotic pathways allow senescent cells to accumulate within tissues, such as the joints, and thus present numerous potential drug targets for OA and other senescence-associated pathologies.
The impact of senescence on OA is complex and just beginning to be understood. Senescence is modulated by a multitude of factors that vary by cell type. The progression of OA involves various cell types within the joint, as well as significant interaction with the innate immune system. Senescent joint cells express high levels of common senescence biomarkers, including p53 and the cyclin-dependent kinase inhibitors p21 and p16INK4a (Figure 1)[17,18]. Joint tissue inflammation is a hallmark of OA, and senescent cells contribute to the inflammatory state via the SASP, in which bioactive molecules, known as SASP-factors, are secreted into the surrounding tissue microenvironment. SASP-factors include pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-8, and tumor necrosis factor (TNF), matrix metalloproteinases (MMPs), microRNAs, growth factors, and small molecule metabolites [19]. Furthermore, there is emerging evidence that different stressors can induce variations in SASP-factor expression [20,21]. In the mouse anterior cruciate ligament transection (ACLT) model of OA, for example, increased Th17 T cell induction of senescence resulted in alterations of Wnt signaling and tissue remodeling [20]. In OA and other aging-related pathologies, the SASP acts to not only promote inflammation, it also propagates senescence by paracrine and autocrine signaling, and can participate in a feed-forward immune response (Figure 1) [20].
Figure 1. Triggers and downstream effects of cellular senescence in the pathogenesis of osteoarthritis.
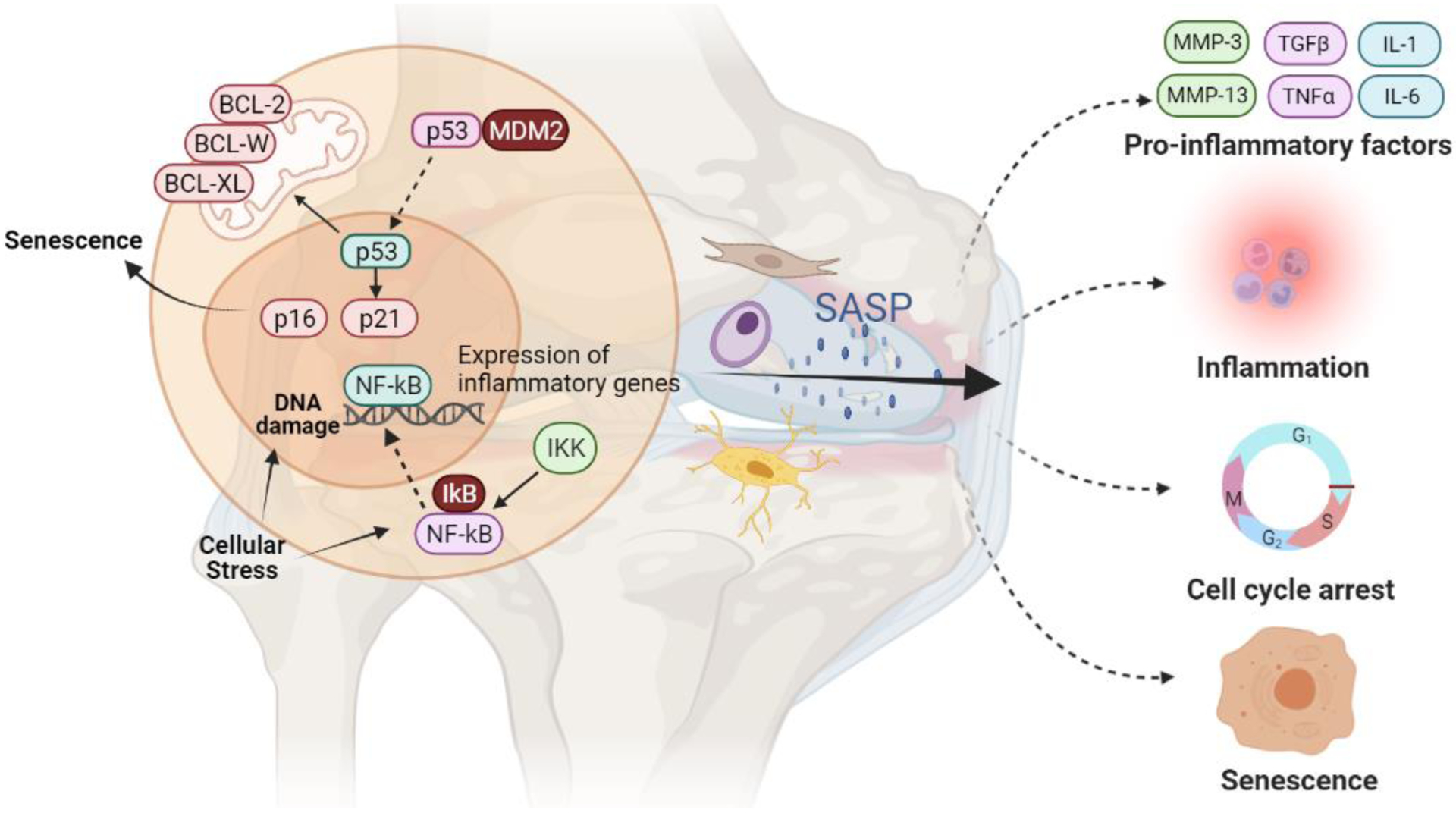
Cellular stress sufficient to induce DNA damage upregulates senescence-associated proteins including p53, p21, and p16. Release of p53 from MDM2 upregulates p21 while p21 and p16 bind to cyclin-dependent kinases causing cell-cycle arrest and senescence. Cellular stress also activates the NFκB pathway through phosphorylation of the inhibitor IkB by IKK which allows NFκB to translocate to the nucleus. NFκB upregulates the expression of inflammatory genes in senescent cells to generate SASP-factors, as well as the expression of intracellular anti-apoptotic proteins including members of the BCL protein family. BCL proteins prevent p53-induced apoptosis by protecting the mitochondrial membrane from permeabilization. SASP factors, which include pro-inflammatory and catabolic OA-mediators, may diffuse into the joint microenvironment from joint tissue cells including chondrocytes, synovial fibroblasts, and perhaps osteocytes, leading to a feed-forward loop of further inflammation, senescence, and tissue degradation. Figure created with BioRender.com.
There are several emerging treatment strategies to target cellular senescence as it relates to OA. These can be grouped into three broad categories: senolytic drugs which counter senescent cell anti-apoptotic pathways to induce apoptosis and clearance of senescent cells, senomorphic drugs which modulate the SASP, and drugs that target the downstream molecules associated with the SASP, in particular cytokines (Table 1). A recent review detailed the mechanistic targets of cellular senescence [22]. Here we build upon that report to provide an overview of the novel therapeutic agents that act at each target, and their potential contributions to the armamentarium against OA.
Table 1.
Therapeutics under evaluation as potential disease-modifying osteoarthritis drugs that may target joint tissue senescence.
| Drug Name | Reference | ||
|---|---|---|---|
| Senolytics | |||
| Dasatinib | BCR-ABL, SRC, c-KIT, ephrin A receptor | Mouse ACLT OA model Phase I clinical trial for IPF and CKD |
28, 29 30, 31 NCT02874989 NCT02848131 |
| Quercetin | PI3K and serpins | Mouse ACLT OA model Phase I clinical trial for IPF and CKD |
28, 29 30, 31 NCT02874989 NCT02848131 |
| UBX-0101 | MDM2 and p53 | Mouse ACLT OA model Phase 1 clinical OA trial Phase 2 clinical OA trial |
12,20 NCT03513016 NCT04129944 |
| Piperlongumine | Oxidation resistance protein OXR1 | In vitro studies Studies of psoriasis In vitro studies of human OA chondrocytes |
32, 33 34 35 |
| Fisetin | PI3K, CDK6, IKK | In vitro human cells Mouse DMM OA model Phase 1 /2 clinical OA trial |
36, 37 38 NCT04210986 |
| Navitoclax, ABT-199, A1331852 | BCL-2, BCL-X1, BCL-W | Mouse DMM OA model | 42–44 |
| Senomorphics | |||
| Ruxolitinib | JAK 1/2 | In vitro inhibition of SASP | 23 |
| Rapamycin | mTOR | Mouse DMM OA model Guinea pig spontaneous OA |
45, 48–49 50 |
| Metformin | AMPK | In vitro increased autophagy Mouse DMM OA model Guinea pig spontaneous OA |
51
52 50 |
| XAV-939 and C113 | Wnt | Mouse DMM model | 55 |
| SM04690 | CLK2 and DYRK1A, Wnt pathway | Phase 2a clinical OA trial Phase 2b clinical OA trial Phase 3 clinical OA trial |
NCT02536833
NCT03122860 NCT04385303 |
| BMS-345541 | IKK | Mouse DMM OA model | 58 |
| Cytokine inhibitors | |||
| IL-17 neutralizing antibody | IL-17 | Mouse ACLT OA model | 20 |
| Lutikizumab | IL-1α/β | Phase 2 clinical OA trial |
62
NCT02087904 |
| Canakinumab | IL-1β | Phase 2 clinical OA trial |
63
NCT04864392 |
| Tocilizumab | IL-6 | Phase 3 clinical OA trial |
65
NCT02477059 |
| Entanercept | TNF-α | Phase 2 clinical OA trial |
66
NCT00394563 |
Senolytic pathways
Senolytics are drugs shown to specifically target and kill senescent cells (SnCs). These therapies reduce the burden of SnCs in aged mice, and improve the microarchitecture of trabecular and cortical bone [23]. An exploration of senolytic pathways as targets against OA gained momentum with the demonstration that selective clearance of SnCs in mice reduced the severity of histologic OA, improved pain behavior, decreased expression of MMP-13 and other SASP factors, and potentially stimulated cartilage regeneration [12]. Furthering support that senescent cells can drive OA pathology, injection of senescent chondrocytes into the knee joints of mice induced osteophyte formation and articular cartilage damage consistent with OA [24]. While it may seem counter-intuitive that eliminating chondrocytes would attenuate cartilage loss, Zhang et al showed that killing chondrocytes in the superficial zone in a surgical model of OA was protective against cartilage damage [25]. Their study, which did not specifically target senescent cells, as well as the subsequent studies of senolytics, suggest that lingering senescent cells and their associated catabolic factors may be more destructive to joint tissues than the removal of those cells via senolytics.
Cancer and aging researchers have elucidated cellular senescence as an anti-apoptotic state with activation of several pro-survival pathways [26,27]. Studies of senolytic therapies for OA have identified numerous small molecule inhibitors which disrupt the pro-survival pathways that are active in SnCs. These pathways include p53, PI3K-AKT, BCL-2, and HSP90 (Table 1). Two of the first small molecules to be studied for their senolytic properties were dasatinib, a tyrosine kinase inhibitor, and quercetin, a flavonoid with antioxidant and estrogenic activities. When administered together, these two drugs reduced the SASP and promoted chondrogenesis in a rat model of distraction arthroplasty performed after ACLT [28]. Studies of the temporomandibular joint provided further evidence that a regimen of dasatinib and quercetin (D+Q) mitigates joint degeneration via the elimination of senescent cells [29]. Zhou et al demonstrated that treatment with D+Q over a six-week interval increased alkaline phosphatase activity, mineralized cartilage, and reduced TMJ degeneration in aged mice (23–24 months)[29]. Human trials of the D+Q regimen to date have been performed for patients with idiopathic pulmonary fibrosis and chronic kidney disease. These phase I clinical trials have demonstrated positive results with decreased SnCs in adipose and epidermal tissue as well as a decrease in SASP factors in plasma [30,31]. Thus far, there are no published trials of these drugs in human OA.
In addition to quercetin, other natural senolytics include piperlongumine and fisetin. Piperlongumine is a compound that can be isolated from the fruit of the long pepper plant. It selectively induces apoptosis in fibroblasts by binding oxidation resistance protein 1 (OXR1)[32,33]. In rats with imiquimod-induced psoriasis, piperlongumine has been shown to reduce serum levels of IL-1β, MMP-1, and MMP-3 [34]. Studies of piperlongumine treatment of human OA chondrocytes have similarly demonstrated reduced nitric oxide, MMP-1, and MMP-13 production in response to IL-1β stimulation [35]. Fisetin is a flavonoid found in several fruits with antioxidant and anti-inflammatory properties. Fisetin is hypothesized to exert an effect via the PI3K/AKT/mTOR and NFκB pathways [36,37]. In mice with OA induced by surgery to destabilize the medial meniscus (DMM), fisetin prevented cartilage damage and inflammation of the synovium [38]. It also decreased IL-6, TNFα, MMP-3, MMP-13, and ADAMTS-5 expression in human OA chondrocytes stimulated by IL-1β. These effects were demonstrated to be mediated by SIRT1 [38]. Clinical trials in humans are ongoing with a phase II trial estimated for completion in 2024 (NCT04770064).
UBX0101 is an inhibitor of the interaction between MDM2 and p53 which allows p53 to facilitate the induction of apoptosis [12,39]. In vivo studies of intraarticular injections following ACLT in mice, found that serial UBX0101 injections increased the clearance of SnCs and reduced OA severity in young animals [20]. However, in mice aged 18 months, UBX0101 injections given after ACLT surgery did not reduce OA severity [20]. In a Phase 1 clinical trial (NCT03513016), UBX0101 reduced joint pain and improved function in OA patients. These results have only been reported thus far in abstract form. Initial data from a Phase 2 trial (NCT04129944), did not meet the primary objective of pain modification at 12 weeks following single injection therapy [40]. It is possible that further studies could show clinical benefit with multiple injections of UBX0101, as was seen in preclinical models, but this approach is not being pursued at this time.
The BCL family proteins are another promising target of senolytic drugs. BCL-2, BCL-X1, and BCL-W are anti-apoptotic proteins which act by inhibiting proteins that destabilize the mitochondrial membrane [41]. The BCL-2, BCL-X1, and BCL-W inhibitor, Navitoclax (ABT-263), has been shown to induce apoptosis in senescent cells and protect against articular cartilage damage in rats in the DMM OA model [42]. A concerning adverse effect noted with ABT-263 injection was the development of severe thrombocytopenia. Other BCL protein inhibitors such as ABT-199 and A1331852 have demonstrated less risk of adverse events and these may prove to have greater potential as human therapies [43,44].
Senomorphic pathways
Rather than enhance the clearance of SnCs, senomorphic drugs modulate and reduce the inflammatory secretions of senescent cells by targeting pathways involved in the SASP. SASP-factors include hundreds of proteins and small molecules that vary by cell-type and inducing stressor but include proinflammatory cytokines and matrix degrading MMPs. While the SASP can be beneficial in the context of tissue remodeling and repair, its paracrine and autocrine effects may contribute significantly to the induction and progression of OA [6,22,23]. Further characterization of the SASP and the pathways that regulate it are a priority for the development of therapies to counter the negative effects of SnCs. SASP regulatory pathways that are currently under investigation include JAK/STAT, mTOR, MAPK, Wnt, and NFκB among others [5,45–47]. Drugs that inhibit or modify these pathways are showing early potential as OA treatments in preclinical studies.
Ruxolitinib is a JAK1/2 inhibitor which is FDA approved for the treatment of rheumatoid and psoriatic arthritis [46]. More recently, ruxolitinib has been investigated for its inhibition of the SASP factors with downregulation of IL-6, TNFα, MMP-1, MMP-3, and MMP-13 in bone marrow stromal cells taken from aged mice [23].
Rapamycin is an inhibitor of mTORC1 and an FDA approved medication for the rejection of organ transplants. mTORC1 regulates SASP by differentially regulating the translation of MAP kinase activated protein kinase 2 (MAPKAPK2) and IL-1α. MAPKAPK2 deactivates ZFP36L1, a zinc finger protein that degrades the mRNA of SASP factors. By inhibiting mTORC1, rapamycin can effectively reduce the stability of SASP mRNA. Dhanabalan et al demonstrated that rapamycin can be delivered to chondrocytes using PLGA microparticles, and in such a system, SASP factors were significantly reduced [45]. Intraperitoneal [48] or intra-articular [49] injection of rapamycin has been shown to reduce OA severity in young mice in the DMM OA model, although rapamycin made spontaneous OA in guinea pigs worse when given in food, which the authors attributed to elevations noted in blood glucose [50].
Metformin is a medication that is commonly used in diabetes to assist with glucose control. It is also considered a senomorphic therapy for its ability to activate AMPK signaling and inhibit the mTOR pathway. AMPK activation via metformin increased autophagy and improved repair of damaged cellular matrix [51]. In the mouse DMM model, metformin had chondroprotective effects and early data suggested that this finding was mediated by AMPK [52]. However, metformin combined with rapamycin did not reduce OA severity in the guinea pig study mentioned above [50].
IL-17 and Wnt signaling are under evaluation as senomorphic targets as they can enhance the production of MMP-13 and other OA mediators, which promote progression of OA [53,54]. Immune profiling has demonstrated alterations in Wnt signaling in fibroblasts with senescence induced by Th17 cells in response to local inflammation [20]. Further experimentation found administration of an IL-17 neutralizing antibody following ACLT in mice decreased MMP-13 and p21. This treatment was protective of tissue structure and improved weight-bearing ability of the affected joint [20]. Additionally, Wnt inhibition with XAV-939 and C113 has been shown to increase anti-catabolic factors and COL2A1 and PRG4 transcripts in OA chondrocytes. Simultaneously, Wnt inhibition ameliorated fibrotic effects of OA fibroblasts by downregulating production of type I collagen [55]. Phase 2 clinical trials have been completed for the Wnt pathway inhibitor lorecivivint (SM04690) that acts by inhibiting the CLK2 and DYRK1A kinases. A phase 2a trial failed to demonstrate improvement in joint space widening or WOMAC pain and function scores compared to placebo injections [2]. However, a phase 2b trial of this drug showed significant benefit in self-reported pain in a subgroup of at risk patients [56].
The NFκB signaling pathway provides further targets for the development of senomorphic DMOADs. Increased numbers of chondrocytes with active NFκB signaling in aged cartilage have been noted and activation of IKKβ accelerated joint degeneration in mice through positive regulation of NFκB [57]. In vitro, IKKβ-NFκB activation in chondrocytes increased the transcripts and protein levels of several SASP-related factors. In mice with an IKKβ gain of function mutation, the transcription factor p50 was found to have a protective affect against cartilage loss, whereas p65 increased the expression of pro-inflammatory factors [57]. Prior studies of NFκκκB signaling and OA demonstrated that inhibition of IKK with BMS-345541 suppresses cartilage degeneration in mice with OA induced by menisci resection [58].
Cytokine inhibitors attenuating down-stream effects of the SASP
Inflammatory cytokines, in particular IL-1α/β, IL-6, IL-8 and TNFα, among others, are increased as a result of the SASP and have been implicated in the pathogenesis of OA [59]. Numerous cytokine inhibitors that are FDA approved for inflammatory diseases such as rheumatoid arthritis (RA) are currently being studied for their potential impact on OA. In vivo experimentation with IL-1 blockade and or IL-1 knockout in mice have failed to demonstrate reduction in cartilage destruction or synovial inflammation in collagenase-induced OA [60]. Regardless, these drugs remain intriguing candidates for OA therapies given their demonstrated safety profile and efficacy with use in several hereditary autoinflammatory conditions including Still’s disease, crystal arthropathy, and Behcet’s disease [61]. IL-1β inhibitors currently undergoing evaluation are lutikisumab and canakinumab. Lutikizumab inhibits both IL-1α and IL-1β but did not improve synovitis or pain associated with knee OA in a phase 2 trial [62]. Canakinumab is a selective IL-1β inhibitor currently under phase 2 clinical trial for patients with knee OA (NCT04864392). The large Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) found that IL-1β inhibition with canakinumab was associated with a significant reduction in serum C-reactive protein (CRP) and lower risk of cardiovascular events in individuals with prior myocardial infarction and elevated baseline CRP [63]. The study also collected data on hip and knee replacement among participants, and post-hoc analysis revealed reduced rates of OA symptoms and OA related joint replacement in participants randomized to canakinumab when compared to placebo [64]. Tocilizumab is an IL-6 inhibitor currently approved for treatment of RA. A phase 3 clinical trial of tocilizumab in participants with hand OA indicated no benefit on pain or any secondary outcomes while adverse events were slightly more frequent [65]. Etanercept is a TNFα inhibitor commonly used for RA and spondyloarthropathies. A multicenter trial did not show reduction in pain or synovitis in participants with hand OA [66].
Conclusions and Future Directions
OA is a debilitating condition with significant global impact. Cellular senescence and the accompaning SASP have emerged as potential key contributors to the deleterious effects of OA on cartilage, synovium and possibly bone. In vivo and in vitro studies have elucidated a wide array of molecular targets to eliminate senescent cells or reduce the effects of the SASP. Many of these targets have yielded therapies undergoing clinical trials. Further innovative studies have led to the discovery and development of therapeutics such as the selective BCL protein inhibitors and heat shock protein inhibitors. The rapid growth of this field demonstrates the potential for future DMOADs that target senescent cells or the products of senescent cells. More research is needed to better understand the factors that promote the SASP in senescent cells. There also remains considerable research to be done towards elucidating the long-term efficacy of these interventions, identifying the OA patient population most likely to respond, and further defining the vast array of mechanistic targets that may yield even more promising therapeutic candidates.
Funding
RL is funded by a grant from the National Institute on Aging (AG044034).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
RL has received consulting fees from Unity Biotechnology and from Benevolent AI.
References
- [1].Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey. Arthritis Care & Research 2016;68:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yazici Y, McAlindon TE, Gibofsky A, Lane NE, Clauw D, Jones M, et al. Lorecivivint, a novel intraarticular CDC-like kinase 2 and dual-specificity tyrosine phosphorylation-regulated kinase 1A inhibitor and Wnt pathway modulator for the treatment of knee osteoarthritis: a phase II randomized trial. Arthritis Rheumatol 2020;72:1694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Conaghan PG, Bowes MA, Kingsbury SR, Brett A, Guillard G, Rizoska B, et al. Disease-modifying effects of a novel cathepsin K inhibitor in osteoarthritis: a randomized controlled trial. Ann Intern Med 2020;172:86–95. [DOI] [PubMed] [Google Scholar]; This phase IIa clinical trial demonstrated that daily oral administration of 100mg of MIV-711, a cathepsin K inhibitor, significantly reduced femoral cartilage thinning over a treatment period of 26 weeks. Cathepsin K is an osteolytic protease released by osteoclasts that is also found in cartilage. This trial indicated a good safety profile of this treatment, with no therapy-related severe adverse events reported. However, the expected outcome of the study- reduction in WOMAC scores of pain, function, and stiffness was not met. Of note, participants in this study were permitted to continue using their baseline analgesic agents.
- [4].Eckstein F, Hochberg MC, Guehring H, Moreau F, Ona V, Bihlet AR, et al. Long-term structural and symptomatic effects of intra-articular sprifermin in patients with knee osteoarthritis: 5-year results from the FORWARD study. Ann Rheum Dis 2021: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Deshmukh V, O’Green AL, Bossard C, Seo T, Lamangan L, Ibanez M, et al. Modulation of the Wnt pathway through inhibition of CLK2 and DYRK1A by lorecivivint as a novel, potentially disease-modifying approach for knee osteoarthritis treatment. Osteoarthritis Cartilage 2019; 27:1347–60. [DOI] [PubMed] [Google Scholar]; In vitro study of lorecivivint indicating its interaction with CLK2 and DYRK1A to prevent phosphorylation of SRSF4–6 and FOXO1 respectively. This study further indicated that this interaction within hMSCs induces chrondrocyte differentiation. Inhibition of SIRT and activation of FOXO1 are elucidated as two of the effects of lorecivivint which act to mediate the Wnt pathway and protect chondrocyte function. This study adds important mechanistic insight to support clinical trials of lorecivivint as a DMOAD.
- [6].Di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol 2021;22:75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Coppé J-P, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008;6:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang X-X, He S-H, Liang X, Li W, Li T-F, Li D-F. Aging, cell senescence, the pathogenesis and targeted therapies of osteoarthritis. Frontiers in Pharmacology 2021;12:2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Childs BG, Zhang C, Shuja F, Sturmlechner I, Trewartha S, Fierro Velasco R, et al. Senescent cells suppress innate smooth muscle cell repair functions in atherosclerosis. Nat Aging 2021;1:698–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kozlowski MR. RPE cell senescence: a key contributor to age-related macular degeneration. Med Hypotheses 2012;78:505–510. [DOI] [PubMed] [Google Scholar]
- [11].da Silva PFL, Ogrodnik M, Kucheryavenko O, Glibert J, Miwa S, Cameron K, et al. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 2019;18:e12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jeon OH, Kim C, Laberge R-M, Demaria M, Rathod S, Vasserot AP, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 2017;23:775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that senescent cells have a causative role in the pathogenesis of OA. Senescent cells were selectively removed from mice after ACLT using intraarticular injections of the senolytic, UBX0101 resulting in diminished onset of post-traumatic OA and enhanced cartilage development. Removing senescent cells from cultures of human OA chondrocytes in vitro decreased the expression of the SASP and OA related genes MMP3, IL6, MMP13 and IL1B. Furthermore, in vitro application of UBX0101 allowed for a significant increase in the proliferation of non-senescent cells.
- [13].Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016;15:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 2017;169:132–147.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu S, Liu S, Wang X, Zhou J, Cao Y, Wang F, et al. The PI3K-Akt pathway inhibits senescence and promotes self-renewal of human skin-derived precursors in vitro. Aging Cell 2011;10:661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Soto-Gamez A, Quax WJ, Demaria M. Regulation of survival networks in senescent cells: from mechanisms to interventions. J Mol Bio 2019;431:2629–43. [DOI] [PubMed] [Google Scholar]
- [17].Diekman BO, Sessions GA, Collins JA, Knecht AK, Strum SL, Mitin NK, et al. Expression of p16INK 4a is a biomarker of chondrocyte aging but does not cause osteoarthritis. Aging Cell 2018;17:e12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shimada H, Sakakima H, Tsuchimochi K, Matsuda F, Komiya S, Goldring MB, et al. Senescence of chondrocytes in aging articular cartilage: GADD45β mediates p21 expression in association with C/EBPβ in senescence-accelerated mice. Pathol Res Pract 2011;207:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gasek NS, Kuchel GA, Kirkland JL, Xu M. Strategies for targeting senescent cells in human disease. Nat Aging 2021;1:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Faust HJ, Zhang H, Han J, Wolf MT, Jeon OH, Sadtler K, et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J Clin Invest 2020;130:5493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]; In vitro, Faust et al found that fibroblasts could be induced to enter senescence by th17 lymphocytes. These cells are stimulated by senescent cells and TGFβ. Additionally, this response was enhanced in older mice. They found that clearance of senescent cells with dual administration of senolytics navitoclax and UBX0101 reduced the type 17 immune signatures in young and aged mice with post-traumatic OA, but this only had a therapeutic effect on young mice. Lastly, they determined that IL-4 has a critical role in IL-17 reduction in response to senolytic therapy after ACLT.
- [21].Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M. Unmasking transcriptional heterogeneity in senescent cells. Curr Biol 2017;27:2652–2660.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Coryell PR, Diekman BO, Loeser RF. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat Rev Rheumatol 2021;17:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Farr JN, Khosla S. Cellular senescence in bone. Bone 2019;121:121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J Gerontol 2017;72:780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang M, Mani SB, He Y, Hall AM, Xu L, Li Y, et al. Induced superficial chondrocyte death reduces catabolic cartilage damage in murine posttraumatic osteoarthritis. J Clin Invest 2016.;126:2893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015;14:644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang E Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res 1995;55:2284–92. [PubMed] [Google Scholar]
- [28].Dai H, Chen R, Gui C, Tao T, Ge Y, Zhao X, et al. Eliminating senescent chondrogenic progenitor cells enhances chondrogenesis under intermittent hydrostatic pressure for the treatment of OA. Stem Cell Res Ther 2020;11:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study sought to characterize OA ankles that fail therapy with distraction arthroplasty and found that these joints had significantly higher levels of senescent chondrogenic progenitor cells. This study further discovered that senolytic treatment with D+Q significantly enhanced chondrogensis induced by distraction arthroplasty in vivo in rat models of traumatic OA. These findings support the role of senescence in the pathogenesis of OA, as well as offer a potentially beneficial treatment strategy of combined arthroplasty and senolytics.
- [29].Zhou Y, Al-Naggar IMA, Chen P-J, Gasek NS, Wang K, Mehta S, et al. Senolytics alleviate the degenerative disorders of temporomandibular joint in old age. Aging Cell 2021;20:e13394. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhou et al demonstrated that D+Q can reduce TMJ degeneration in aged mice (23–24 months). They found that 6 weeks of bi-weekly administration of D+Q increased alkaline phosphatase activity and mineralized cartilage, while significantly reducing OARSI scores in old mice. They further showed that the increase in mineralized cartilage did not result in encroachment on non-mineralized cartilage. These effects were not seen in young mice which further supports the possibility that D+Q mitigates joint degeneration via the elimination of senescent cells.
- [30].Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019;40:554–63. 10.1016/j.ebiom.2018.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hickson LJ, Prata LGPL, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019;47:446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang X, Zhang S, Liu X, Wang Y, Chang J, Zhang X, et al. Oxidation resistance 1 is a novel senolytic target. Aging Cell 2018;17:e12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging 2016;8:2915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Thatikonda S, Pooladanda V, Sigalapalli DK, Godugu C. Piperlongumine regulates epigenetic modulation and alleviates psoriasis-like skin inflammation via inhibition of hyperproliferation and inflammation. Cell Death Dis 2020;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hu Y, Yan J. Piperlongumine attenuates IL-1β-induced inflammatory response in chondrocytes. Biomedicalresearch 2018;29: 0976–1683e. [Google Scholar]
- [36].Khan N, Afaq F, Khusro FH, Mustafa Adhami V, Suh Y, Mukhtar H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int J Cancer 2012;130:1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol 2007;71:1703–14. [DOI] [PubMed] [Google Scholar]
- [38].Zheng W, Feng Z, You S, Zhang H, Tao Z, Wang Q, et al. Fisetin inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Inter Immunopharm 2017;45:135–47. [DOI] [PubMed] [Google Scholar]
- [39].Thoppil H, Riabowol K. Senolytics: a translational bridge between cellular senescence and organismal aging. Front Cell Dev Biol 2020;7:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dolgin E Send in the senolytics. Nat Biotechnol 2020;38:1371–7. [DOI] [PubMed] [Google Scholar]
- [41].Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008;9:47–59. [DOI] [PubMed] [Google Scholar]
- [42].Yang H, Chen C, Chen H, Duan X, Li J, Zhou Y, et al. Navitoclax (ABT263) reduces inflammation and promotes chondrogenic phenotype by clearing senescent osteoarthritic chondrocytes in osteoarthritis. Aging 2020;12:12750–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang L, Doherty GA, Judd AS, Tao Z-F, Hansen TM, Frey RR, et al. Discovery of A-1331852, a First-in-Class, Potent, and Orally-Bioavailable BCL-XL Inhibitor. ACS Med Chem Lett 2020;11:1829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]; This publication describes the use of structure-based design to create an orally bioavailable selective BCL-Xl inhibitor. This molecule, A-1331852, was tested in vivo in rat xenograft models of human colorectal cancer and demonstrated significant efficacy in reducing tumor volume.
- [44].Cang S, Iragavarapu C, Savooji J, Song Y, Liu D. ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. J Hematol Oncol 2015;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dhanabalan KM, Gupta VK, Agarwal R. Rapamycin–PLGA microparticles prevent senescence, sustain cartilage matrix production under stress and exhibit prolonged retention in mouse joints. Biomater Sci 2020;8:4308–21. [DOI] [PubMed] [Google Scholar]
- [46].Fragoulis GE, McInnes IB, Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology 2019;58:i43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wan M, Gray-Gaillard EF, Elisseeff JH. Cellular senescence in musculoskeletal homeostasis, diseases, and regeneration. Bone Res 2021;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Caramés B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis 2012;71:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Takayama K, Kawakami Y, Kobayashi M, Greco N, Cummins JH, Matsushita T, et al. Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res Ther 2014;16:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Minton DM, Elliehausen CJ, Javors MA, Konopka AR. Rapamycin induced hyperglycemia is associated with exacerbated age-related osteoarthritis Arthritis Res Ther. 2021; 23(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016;12:412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Li J, Zhang B, Liu W-X, Lu K, Pan H, Wang T, et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann Rheum Dis 2020;79:635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of metformin as a potential therapy for OA used the DMM murine model of OA to show beneficial effect of metformin given either before or after surgery. The positive effects of metformin were not seen in AMPKα1 knock out mice, indicating a significant role of AMPK signaling in the chondroprotective effects of metformin. This study provides important insight towards metformin as a DMOAD and its likely mechanism of action.
- [53].Ruan G, Xu J, Wang K, Wu J, Zhu Q, Ren J, et al. Associations between knee structural measures, circulating inflammatory factors and MMP13 in patients with knee osteoarthritis. Osteoarthritis and Cartilage 2018;26:1063–9. [DOI] [PubMed] [Google Scholar]
- [54].Wang Y, Fan X, Xing L, Tian F. Wnt signaling: a promising target for osteoarthritis therapy. Cell Commun Signal 2019;17:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lietman C, Wu B, Lechner S, Shinar A, Sehgal M, Rossomacha E, et al. Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight 2018;3:96308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yazici Y, McAlindon TE, Gibofsky A, Lane NE, Lattermann C, Skrepnik N, et al. A Phase 2b randomized trial of lorecivivint, a novel intra-articular CLK2/DYRK1A inhibitor and Wnt pathway modulator for knee osteoarthritis. Osteoarthr Cartil 2021;29:654–66. [DOI] [PubMed] [Google Scholar]
- [57].Catheline SE, Bell RD, Oluoch LS, James MN, Escalera-Rivera K, Maynard RD, et al. IKKβ–NFκB signaling in adult chondrocytes promotes the onset of age-related osteoarthritis in mice. Sci Sig 2021;14:eabf3535. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, Catheline et al characterized the regulation of NFκB signaling by subunits, p65 and p50 as well as the role of the IκB kinase complex. Histology of knee joints from aged mice revealed concomitant reduced IκBα inhibition of NFκB as well as loss of cartilage consistent with OA. Activation of NFκB through expression of constitutively active inhibitor of IκB kinase β (IKKβ) was shown to enhance the progression of OA. Loss of the p50 subunit further contributed to accelerated onset of an OA-phenotype. In vitro, activation of IKKβ stimulated expression of the SASP and loss of p65 diminished the expression of this phenotype. Catheline et al conclude that IKKβ and p65 play an early role in the development of SASP in chondrocytes and contribute to onset of an OA-like phenotype, while p50 may have protective effects.
- [58].Murahashi Y, Yano F, Kobayashi H, Makii Y, Iba K, Yamashita T, et al. Intra-articular administration of IκBα kinase inhibitor suppresses mouse knee osteoarthritis via downregulation of the NFκB/HIF-2α axis. Sci Rep 2018;8:16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Van den Bosch MH, Van Lent PL, Van der Kraan PM. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthr Cartil 2020;28:532–43. [DOI] [PubMed] [Google Scholar]
- [60].Nasi S, Ea H-K, So A, Busso N. Revisiting the role of interleukin-1 pathway in osteoarthritis: interleukin-1α and −1β, and NLRP3 inflammasome are not involved in the pathological features of the murine menisectomy model of osteoarthritis. Front Pharm 2017;8:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dayer J-M, Oliviero F, Punzi L. A brief history of IL-1 and IL-1ra in rheumatology. Front Pharm 2017;8:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Fleischmann RM, Bliddal H, Blanco FJ, Schnitzer TJ, Peterfy C, Chen S, et al. A phase II trial of Lutikizumab, an anti-interleukin-1α/β dual variable domain immunoglobulin, in knee osteoarthritis patients with synovitis. Arthritis Rheumatol 2019;71:1056–69. [DOI] [PubMed] [Google Scholar]
- [63].Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. NEJM 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- [64].Schieker M, Conaghan PG, Mindeholm L, Praestgaard J, Solomon DH, Scotti C, et al. Effects of interleukin-1β inhibition on incident hip and knee replacement. Ann Intern Med 2020;173:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Richette P, Latourte A, Sellam J, Wendling D, Piperno M, Goupille P, et al. Efficacy of tocilizumab in patients with hand osteoarthritis: double blind, randomised, placebo-controlled, multicentre trial. Ann Rheum Dis 2021;80:349–55. [DOI] [PubMed] [Google Scholar]
- [66].Kloppenburg M, Ramonda R, Bobacz K, Kwok W-Y, Elewaut D, Huizinga TWJ, et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2018;77:1757–64. [DOI] [PubMed] [Google Scholar]


