Figure 5. SPARK regulates egress and invasion through modulation of intracellular Ca2+ stores.
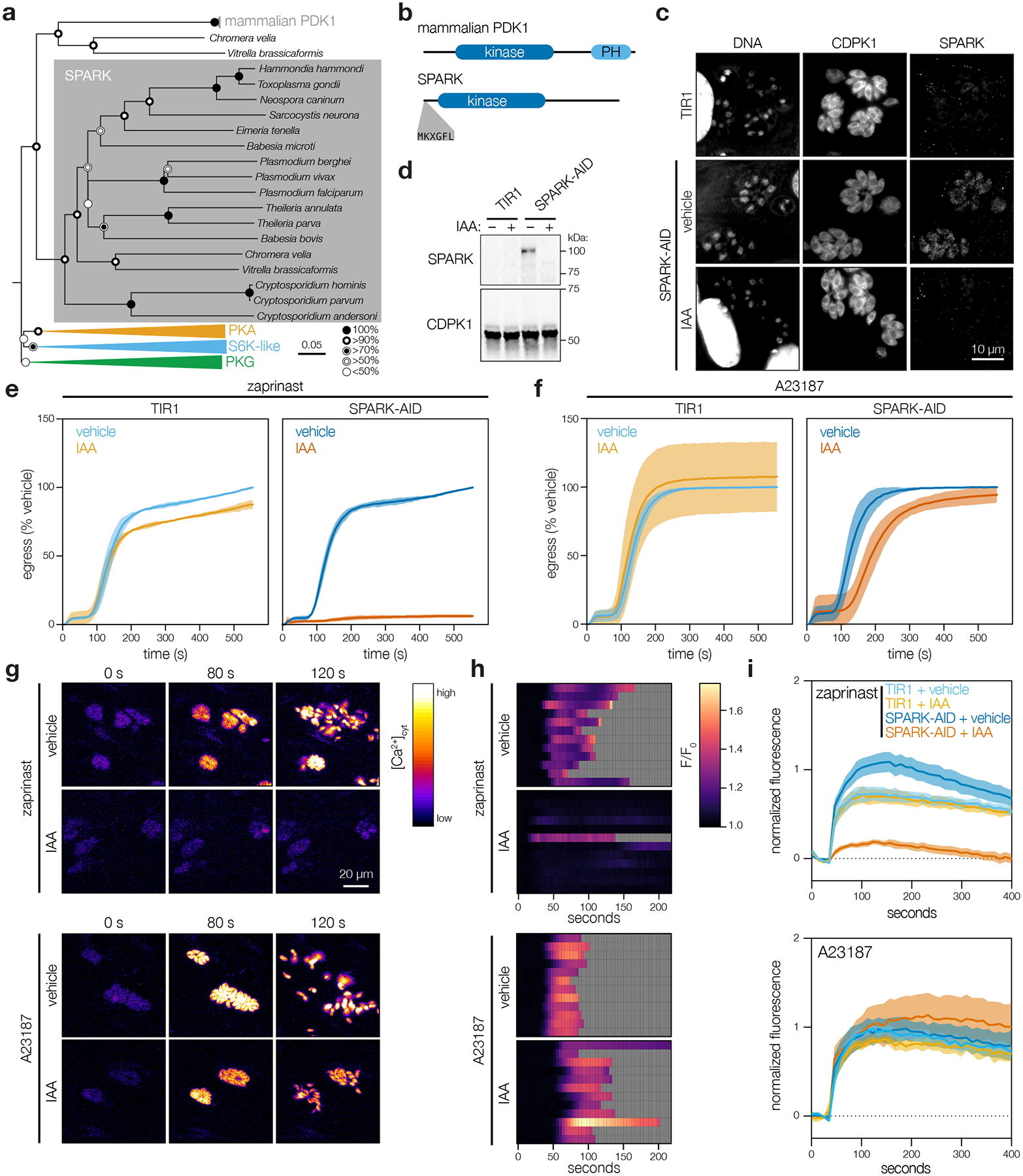
a, Neighbor-joining phylogenetic tree of kinase domains from representative apicomplexan species, along with mammalian PDK1 orthologues and related AGC kinases. Bootstraps determined from 1000 simulations. Scale indicates substitutions per site. b, Models of the canonical mammalian PDK1 and the apicomplexan SPARK proteins. The kinase domains, mammalian pleckstrin homology (PH) domain, and conserved apicomplexan MKXGFL motif are shown. c, SPARK-AID was visualized by immunofluorescence microscopy and immunoblotting using the V5 epitope. SPARK-AID was undetectable after 24 h of IAA treatment. Staining for CDPK1 was used to identify parasites, and nuclei were stained with DAPI. Channels adjusted equivalently across all samples. d, SPARK-AID depletion, as in c, monitored by immunoblot. SPARK-AID is expected to run at 98 kDa. e–f, Parasite egress stimulated with zaprinast (e) or the Ca2+ ionophore A23187 (f) following 24 h of treatment with vehicle or IAA. Egress was monitored by the number of host cell nuclei stained with DAPI over time. Mean graphed for n = 3 biological replicates. Shaded regions represent ± S.D. g, Selected frames from live video microscopy of zaprinast- or A23187-stimulated SPARK-AID parasites expressing the genetically encoded Ca2+ sensor GCaMP6f. Parasites were grown for 24 h with vehicle or IAA prior to the stimulation of egress. h, Kymographs showing normalized fluorescence per vacuole relative to the initial intensity, for 12 vacuoles per strain from the experiments in g. Gray areas represent the period following egress of the vacuole under observation. i, Extracellular parasites in basal Ca2+ buffer stimulated with zaprinast or the Ca2+ ionophore A23187, following 24 h of treatment with vehicle or IAA. Cytosolic Ca2+ flux was measured in bulk as GCaMP6f fluorescence normalized to the initial and maximum fluorescence following aerolysin permeabilization in 2 mM Ca2+. Mean ± S.E. graphed for n = 3–6 biological replicates.
