Abstract
Background
Salvage conventional chemotherapy followed by high-dose therapy (HDT) and autologous stem cell transplantation (ASCT) is the treatment of choice for most patients with refractory classical Hodgkin lymphoma (cHL). In the era of pandemic COVID-19, there are obstacles to administering salvage chemotherapy followed by HDT and ASCT due to side effects and toxicities which make the patient more susceptible to COVID-19 infection. The toxicities and side effects of BV are different from salvage chemotherapy, but it has clear efficacy as monotherapy.
Case Presentation
A 46-year-old female with a history of cHL nodular sclerosis subtype was presented with right cervical lymph node enlargement, after 3 cycles of first-line chemotherapy ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) 3 months ago. She was afraid to undergo second-line chemotherapy in the era of pandemic COVID-19 because of the side effects and toxicities; therefore, she was given 8 cycles of BV as monotherapy. The response of the treatment was complete remission.
Conclusion
In this particular case of patient, BV as monotherapy can be an option during the pandemic COVID-19 for refractory cHL ineligible for second-line chemotherapy followed by HDT and ASCT.
Keywords: brentuximab vedotin, COVID-19, monotherapy, refractory Hodgkin’s lymphoma
Introduction
The vast majority of patients with classical Hodgkin lymphoma (cHL) are cured with combination first-line chemotherapy with or without radiotherapy. Approximately 10–30% of patients will be relapsed/refractory within 5 years thereafter.1 Salvage conventional chemotherapy followed by high-dose therapy (HDT) and autologous stem cell transplantation (ASCT) is the treatment of choice for most patients with relapsed cHL.2
Brentuximab vedotin (BV) is an antibody–drug conjugate (ADC) consisting of an anti-CD30 monoclonal antibody conjugated to an anti-microtubule agent, monomethyl auristatin E (MMAE). Early trials of BV were predominantly conducted in heavily pretreated patients and the success leads to the evaluation of the efficacy and the safety of BV in a range of situations and the patients.3 In this case, we would like to report the benefit of BV as a second-line treatment of refractory Hodgkin lymphoma (HL) in the era of pandemic COVID-19.
Case Presentation
A 46-year-old female with a history of classical HL nodular sclerosis subtype complained of a right cervical lymph node enlargement after 3 cycles of first-line chemotherapy ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) three months ago. There were no fever, night sweats, and weight loss. She had no other pre-existing illness. On physical examinations, we found an enlargement of multiple right anterior cervical lymph nodes. No tenderness and no signs of inflammation.
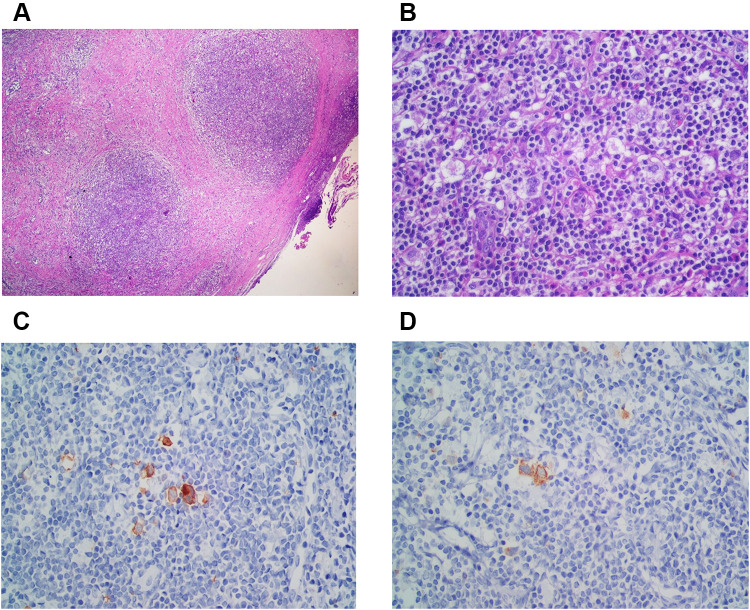
The surgeon performed an excisional biopsy to confirm the refractory of the disease. The cervical lymph node biopsy specimen (Hematoxylin & Eosin staining (H&E)) showed lymph nodes with nodular growth pattern surrounded by collagen bands (Figure 1A). There were Hodgkin Reed-Sternberg cells, lacunar cells, small lymphocytes, and other inflammatory cells as background (Figure 1B). The immunohistochemistry (IHC) showed positive for CD 30 (Figure 1C), CD 15 (Figure 1D), and MUM1, dim positive for PAX. The morphological features and IHC result confirmed the classical HL nodular sclerosis subtype; therefore, we established the diagnosis of refractory nodular sclerosis HL.
Figure 1.
Nodular sclerosis classic Hodgkin lymphoma. (A) Nodular growth pattern surrounded by fibrous collagen bands (H&E, 40x). (B) Lacunar cells were present with inflammatory cells as background (H&E, 400x). (C) CD 30 positive (IHC, 400x). (D) CD 15 positive (IHC, 400x).
We performed 18-Fluorodeoxyglucose positron emission tomography (PET) scan for staging and revealed lymph nodes involvement of right inferior jugular, right posterior cervical, right supraclavicular, right superior paratracheal, aorta para-arcus, aortopulmonary window, upper lobe of the left lung, peritoneum, and mesenterial with diffuse metabolic activity of all bone marrow. These findings are suggested as an extranodal extension. We did not find metastatic lung disease. Clinical staging was restaged with Clinical Stage (CS) IV according to the Ann-Arbor staging system.
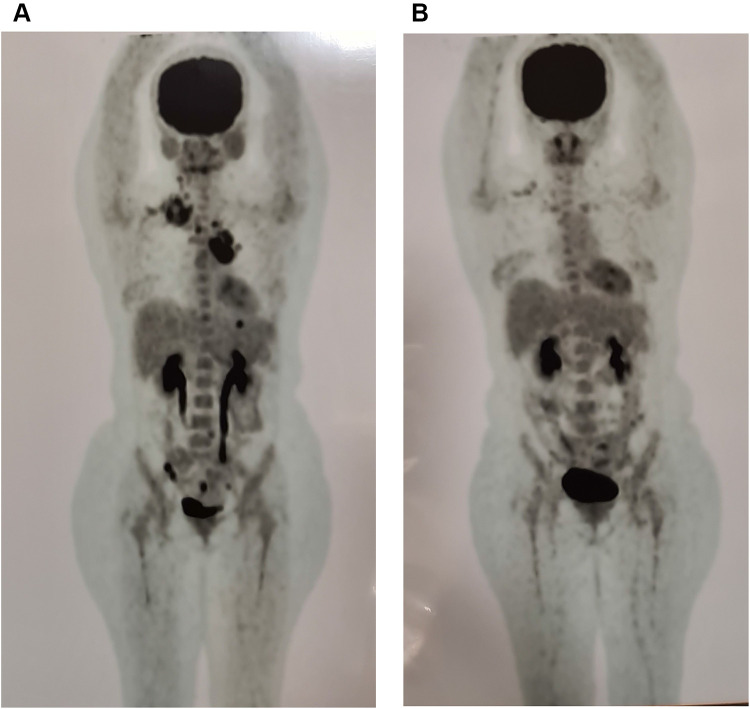
We offered salvage treatment with conventional chemotherapy with DHAP followed by HDT and ASCT; however, the patient hesitated since she was worried about the toxicities which could make her more susceptible to getting COVID-19 infection in the era of the COVID-19 pandemic. After discussion and explanation to the patient and the family about the efficacy and side-effect profile of BV, finally, the patient chose BV for second-line treatment to control the tumor. We administered BV monotherapy 1.8 mg/m2 every 3 weeks for up to 16 cycles. The patient tolerated BV well for eight cycles without any significant toxicities and side effects. We stopped the administration of BV because the patient had already reached remission after 8 cycles, and she was not willing to continue the chemotherapy until 16 cycles. The PET scan before and after eight cycles of BV are shown in Figures 2A and B, respectively. The evaluation of the PET scan after the end of cycle-8 showed complete remission (Figure 2B). We then suggested the patient to control every 3 months to observe the remission status with HDT and ASCT as an option to salvage if the cHL further relapsed.
Figure 2.
(A) PET scan before chemotherapy showed prominent involvement of right inferior jugular, right posterior cervical, right supraclavicular, and right superior paratracheal lymph nodes; para-arcus aorta, aortopulmonary window, upper lobe of left lung, peritoneum, and mesenterial lymph nodes with diffuse metabolic activity of all bone marrow, (B) PET scan after 8 cycles of BV showed complete remission of the tumor.
Discussion
Following frontline treatment failure, the majority of patients with relapsed/refractory cHL who are transplant candidates require salvage chemotherapy, for example, DHAP (dexamethasone, high dose cytarabine, and cisplatin) or ICE (Ifosfamide, Carboplatin, and Etoposide), followed by HDT and ASCT. However, a minority, those aged >65–70 years or with serious comorbidities are not candidates for ASCT.2,5
The United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved BV for treatment of HL after the failure of ASCT or after the failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates and consolidation treatment after ASCT in patients at risk of relapse or progression.4 The role of BV in the first-line therapy for non-transplant candidates was evaluated in a Phase II trial. When BV was administered to 27 patients with HL that were ≥60 years old and ineligible for conventional combination chemotherapy, 19 and 5 patients achieved complete response (CR) and partial response (PR), respectively, with a median duration of response of 9.1 months (range, 2.8–20.9). Several studies have reported the outcomes of compassionate use of BV and retrospective data. In general, real-world data provided similar clinical outcomes to the pivotal phase II trial, with a CR rate of 18%–46% and PFS between 5.7 months and 10.2 months.3 BV has not been approved as second-line therapy of relapsed cHL ineligible for salvage chemotherapy followed by HDT and ASCT but might be applicable as an off-label option.5 Nagashima et al reported a case of successful BV monotherapy against late relapse of cHL 6 years after the first remission.6 Wu et al reported a case in which BV was a treatment for primary refractory Hodgkin lymphoma.7 Alessandro et al reported that BV or immune checkpoint inhibitors (every 4 weeks) can be used for relapsed or refractory cHL during the pandemic,8 and Pietro et al reported that BV as a single agent may be considered as salvage (depending upon accessibility) prior to ASCT, during the COVID-19 pandemic.9 Another study in Asia reported that BV is a feasible treatment option for R/R Hodgkin Lymphoma with tolerable toxicity in Asian patients, and those who achieved PR could be cured without further transplantation.10 Progression-free survival (PFS) rates of BV in 1 and 2 years were reported 63.2% and 45.2%, respectively, with the median PFS of 1.38 years.11
This patient was worried to participate in salvage chemotherapy followed by HDT and ASCT, even she was afraid to undergo second-line chemotherapy in the era of pandemic COVID-19 because of side effects and toxicities which can make her more susceptible to getting the COVID-19 infection. Finally, after discussion, the patient preferred to choose BV as second-line therapy for refractory cHL. After 8 cycles of BV monotherapy, the response of treatment was complete remission. In this case, we only administered 8 cycles of BV because the patient had already reached remission and she was hesitant to continue the chemotherapy until 16 cycles. The patient tolerated BV well with minimal peripheral neuropathy and mild neutropenia and good quality of life. We are still monitoring the patient’s condition, and there are no signs of relapse until now.
Conclusion
In this particular case of patient with refractory cHL ineligible for second-line chemotherapy followed by HDT and ASCT, BV monotherapy can be an option during the pandemic era of COVID-19. BV monotherapy showed complete remission and was well tolerated with minimal side effects, which was peripheral neuropathy.
Ethical and Consent Statements
Written informed consent was obtained from the patient for anonymized patient information to be published in this case report. Ethical approval was obtained from Metropolitan Medical Centre Hospital’s ethics committee to publish this case report.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Van Hende V, Verhoef G, Snauwaert S, et al. Hodgkin’s lymphoma: Belgian hematology society guidelines in diagnosis, treatment, and follow-up. Belg J Hematol. 2018;9(6):214–224. [Google Scholar]
- 2.Eichenauer DA, Aleman BMP, Andre M, et al. Hodgkin lymphoma: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2018;29(Supplement4):iv19–iv29. doi: 10.1093/annonc/mdy080 [DOI] [PubMed] [Google Scholar]
- 3.Yi JH, Kim SJ, Kim WS. Brentuximab vedotin: clinical update and practical guidance. Blood Res. 2017;52(4):243–253. doi: 10.5045/br.2017.52.4.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelo R, McGinn K, Kwitkowski V, et al. US food and drug administration approval summary: brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma. Clin Cancer Res. 2012;18(21):5845–5849. doi: 10.1158/1078-0432.CCR-12-1803 [DOI] [PubMed] [Google Scholar]
- 5.Vassilakopoulos TP, Asimakopoulos JP, Konstantopoulos K, Angelopoulou MK. Optimizing outcome in relapsed/ refractory Hodgkin lymphoma: a review of current and forthcoming therapeutic strategies. Ther Adv Hematol. 2020;11:1–31. doi: 10.1177/2040620720902911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagashima K, Kikuchi S, Iyama S, et al. Successful brentuximab vedotin monotherapy against late relapse of classical Hodgkin lymphoma 6 years after first remission. Clin Case Rep. 2020;00:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu HB, Yeh SA, Chen HY. Brentuximab vedotin treatment for primary refractory Hodgkin lymphoma. Case Rep Hematol. 2013;2013:1–5. doi: 10.1155/2013/351292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isadori A, de Lleval L, Gergis U, et al. Management of patients with hematologic malignancies during the COVID-19 pandemic: practical considerations and lessons to be learned. Front Oncol. 2020;10:1439. doi: 10.3389/fonc.2020.01439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pietro DC, Georgia M, Judith T, et al. Australian and New Zealand consensus statement on the management of lymphoma, chronic lymphocytic leukaemia and myeloma during the COVID-19 pandemic. Intern Med J. 2020;03:1–13. [DOI] [PubMed] [Google Scholar]
- 10.Tien FM, Tsai CH, Liu JH, Lin CT. Brentuximab vedotin as a salvage treatment for relapsed and refractory Hodgkin lymphoma patients in Taiwan. J Formos Med Assoc. 2019;118(10):1466–1470. doi: 10.1016/j.jfma.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 11.Král Z, Michalka J, Móciková H, et al. Treatment of Relapsed/Refractory Hodgkin Lymphoma: real-World Data from the Czech Republic and Slovakia. J Cancer. 2019;10(21):5041–5048. doi: 10.7150/jca.29308 [DOI] [PMC free article] [PubMed] [Google Scholar]