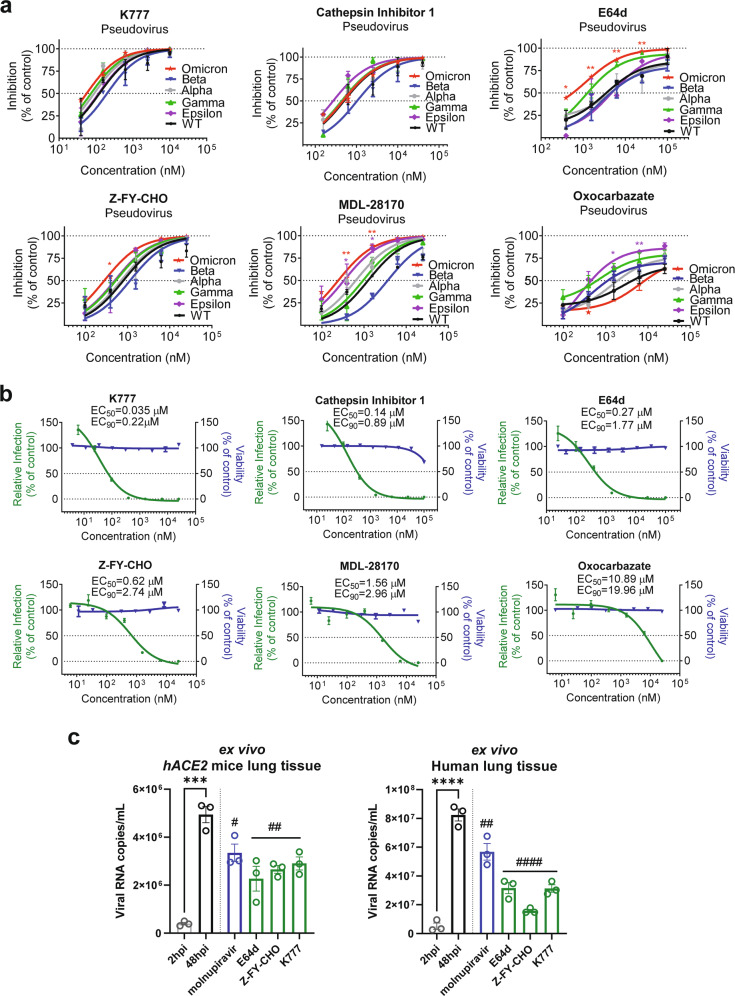
Fig. 4. CTSL inhibitors prevent infection with SARS-CoV-2 and mutant variant PsVs in vitro.
a Vero E6 cells were pretreated with increasing concentrations of each compound for 16 h and were then infected with different SARS-CoV-2 variant PsVs as indicted. At 24 hpi, infectivity was measured by a luciferase assay. The data were normalized to the average value in vehicle-treated cells and are shown as inhibition rates (n = 3). Statistical significance was assessed between the indicated variant and WT PsV by two-way ANOVA with Dunnett’s post-hoc test. The data are presented as the means ± SEM. *P < 0.05, **P < 0.01. b Vero E6 cells were pretreated with increasing concentrations of each compound for 16 h and were then infected with SARS-CoV-2 at an MOI of 0.01. At 24 h post-infection, viral RNA copies in supernatants were quantified by RT-qPCR. The data were normalized to the average value in vehicle-treated cells and are shown as relative infection percentages. The EC50 values for each compound are indicated. Cell viability was evaluated with a CCK kit (TransGen Biotech) (n = 3). c Ex vivo lung tissues from hACE2 mice or a human donor were infected with SARS-CoV-2 with an inoculum of 1 × 106 PFU/mL for 2 h. Then the inoculum was removed and changed with medium with indicated compounds (10 μM for molnupiravir, 4 μM for E64d, 5 μM for Z-FY-CHO, and 0.4 μM for K777) for another 48 h. Tissues were harvested (without adding compounds) at 2 hpi or 48 hpi to determine the viral growth ability. Tissues were harvested at 48 hpi for quantification of viral RNA (n = 3). Statistical significance was assessed between 2 hpi and 48 hpi by unpaired two-tailed Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Statistical significance was assessed between the indicated drug and 48 hpi by one-way ANOVA with Tukey’s post-hoc test. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001. The data are presented as the means ± SEM.