FIGURE 3.
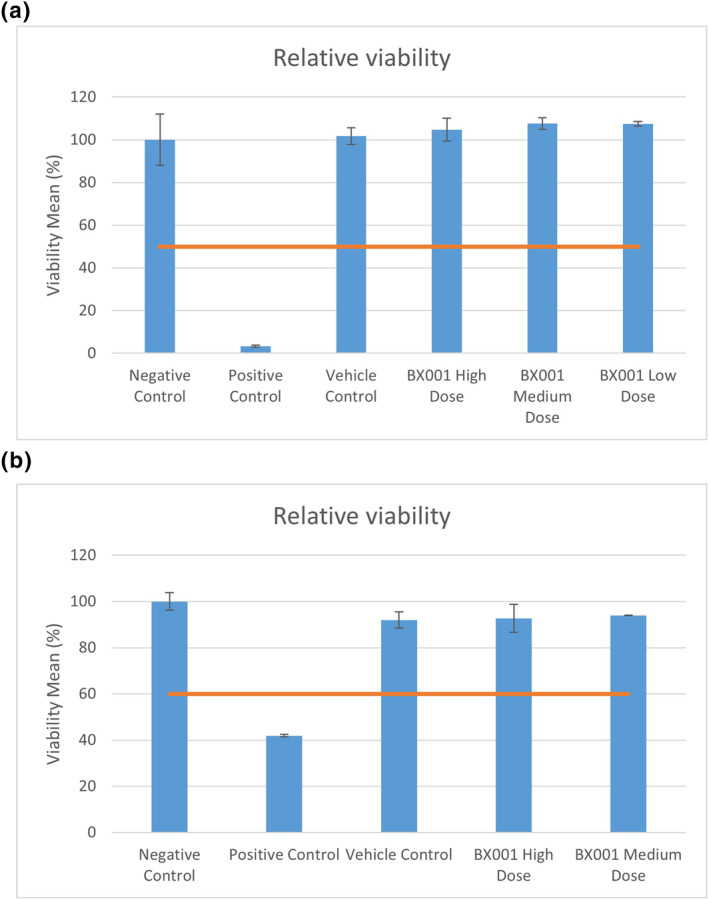
Testing for irritation potential by relative viability after application of BX001 phages to reconstituted human skin and ocular tissue models. (a) Reconstructed human epidermal tissues (EpiDerm™ MatTek) consisting of normal human‐derived epidermal keratinocytes were exposed for 35 min to BX001 phages at 100× (high), 10× (medium) and 1× (low) fold concentrations of the maximal intended dose for human exposure and compared to tissues exposed to negative control (DPBS Rinse Solution, #TC‐PBS, MatTek Corporation), positive control (5% SDS Solution, #TC‐SDS‐1, MatTek Corporation) and vehicle control (SM buffer) with respect to relative viability. Cell viability was measured by MTT [(3‐4,5‐dimethyl thiazole 2‐yl) 2,5‐diphenyltetrazoliumbromide] assay performed per manufacturer’s instructions and with reagents provided by the manufacturer. None of the test items yielded values below 50% relative viability (red line) and they are considered non‐irritants. (b) Reconstructed human cornea‐like epithelium tissues (EpiOcular™ MatTek) were exposed in triplicate to BX001 phages at high (100×) and medium (10×) concentrations and compared to negative control (tissue culture grade water, #03‐055‐1A, Biological Industries), positive control (Methyl acetate, #TC‐MA, MatTek Corporation) and vehicle control (SM Buffer) with respect to relative viability (%) as above. None of the test items yielded results below 60% relative viability (red line) and they are considered non‐irritants. Data represent mean ± SD (standard deviation)
