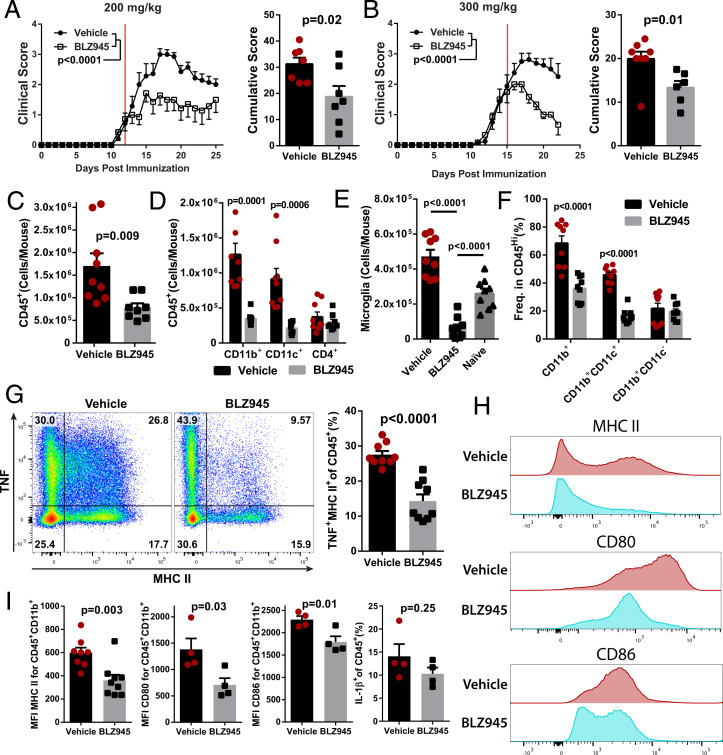
Fig. 3.
BLZ945 suppresses ongoing clinical EAE and reduces the number of myeloid APCs in the CNS. C57BL/6J mice were immunized and allowed to develop clinical signs of EAE before treatment with BLZ945 (n = 7) or vehicle (n = 8). (A) Clinical course and cumulative score for mice treated with 200 mg/kg BLZ945 starting at a clinical score of ∼1. The red line indicates start of treatment. (B) Mice treated with 300 mg/kg BLZ945 (n = 6) or vehicle (n = 8), starting at a clinical score of ∼2. A and B were compiled from two independent experiments. Significance for clinical course determined by two-way repeated measures ANOVA and by unpaired Student’s t test for cumulative scores. (C–I) Analysis of the CNS (pooled brain and spinal cords) by flow cytometry. (C) Number of CD45+ cells. (D) Number of CD45+ cells that also expressed CD11b, CD11c, or CD4. (E) Number of CD45LoCD11b+CX3CR1Hi microglia. Naïve mice were not immunized or otherwise manipulated. (F) Frequency of CD45Hi cells that also express CD11b and/or CD11c. (G) TNF and MHCII expression in CD45+ cells. (H and I) Expression of MHCII, CD80, and CD86 in CD45+CD11b+ cells. Significance for (C–I) was calculated by unpaired Student’s t test. P value corrections for multiple comparisons was performed by false discovery rate approach with Q = 0.01 as a cutoff. Error bars are SEM.