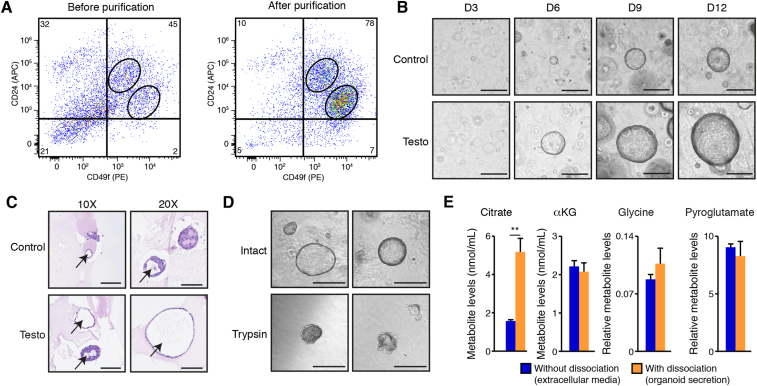
Figure 2.
Primary mouse prostate organoids exhibit the citrate-secretory phenotype. A) Flow cytometry analyses of basal epithelial cells (CD49fhigh/CD24intermediate) and luminal epithelial cells (CD49fintermediate/CD24high) before and after purification. Numbers in the corners are percentages. One representative experiment of two independent experiments is shown. B) Brightfield visualization of organoids over 12 days in three-dimensional culture with and without testosterone, an androgen treatment. Testosterone induces a significant increase in organoid size without changing the number of organoids (quantification of organoid numbers and sizes is available in Supplemental Figure S2C). Testo: Testosterone treatment. Scale bars = 300 μm. C) H&E visualization of mouse prostate organoids after 14 days in culture, with and without testosterone. Arrows show the presence of internal lumen in these organoids. Scale bars = 125 μm and 100 μm, respectively, for 10× and 20× view. Note that during the fixation process, bigger organoids tend to lose their circular architecture. D) Brief treatment with trypsin (3 min) disrupts the three-dimensional architecture of mouse prostate organoids to allow connection of the internal lumen with the extracellular media. Scale bars = 300 μm. E) GC–MS metabolite quantification in extracellular culture media of mouse prostate organoids treated with testosterone, with and without dissociation, as shown in D. With dissociation, this media contains luminal secretion. Results are shown as the average ± SEM of one representative experiment performed in quadruplicates, out of five independent experiments. ∗∗p < 0.01.