Abstract
Background and aims
Recently, there has been a considerable increase in patients with nonalcoholic fatty liver disease. Availability of high-efficacy drugs for hepatitis B and hepatitis C virus (HCV) infection may have changed the disease prevalence. We aimed to study the impact of this changing epidemiology in patients undergoing liver transplantation (LT) over a 10-year period.
Methods
The study population was stratified into Period 1 (2009–2014) and Period 2 (2015–2019). Demographics, indications for LT and changes in the epidemiology between two periods were analysed. Aetiology-based posttransplant survival analysis was carried out.
Results
Indication for LT among 1017 adult patients (277 in Period 1 and 740 in Period 2) showed a significant increase in nonalcoholic steatohepatitis (NASH; 85 [30.7%] and 311 [42%]; P = 0.001), decrease in hepatitis C (49 [17.7%] and 75 [10.1%]; P = 0.002), and increase in hepatocellular carcinoma from Period 1 to Period 2 (13 [26.5%] to 38 [50.7%]; P = 0.009) among HCV patients. Patients transplanted for NASH had a lower 5-year survival compared with viral hepatitis (75.9% vs 87.4%; P = 0.03). There was a strong association between coronary artery disease and NASH (hazard ratio = 1.963, 95% confidence interval, 1.19–3.22).
Conclusion
NASH is the leading indication for liver transplantation in India, surpassing viral hepatitis in recent years.
Keywords: nonalcoholic steatohepatitis, liver transplantation, viral hepatitis, hepatocellular carcinoma, cardiovascular disease
Abbreviations: ASH, Non-alcoholic steatohepatitis; CAD, Coronary artery disease; CLD, Chronic liver disease; DAA, Direct acting antiviral drugs; DM, Diabetes mellitus; HBV, Hepatitis B virus infection; HCC, Hepatocellular carcinoma; HCV, Hepatitis C virus infection; LT, Liver transplantation; NAFLD, Non-alcoholic fatty liver disease; SVR, Sustained virological response
Liver transplantation (LT) is the only curative therapy for patients with end-stage liver disease (ESLD). Nevertheless, there remains a wide geographical variation in the aetiology of chronic liver disease (CLD) across the globe.1 Chronic hepatitis B virus (HBV) infection is more prevalent in Asia and Africa (0.7–22.3%) compared with the West.2 However, chronic hepatitis C virus (HCV) infection prevails between 1% and 4% and is more common in central Asia and Japan.3 Alcohol-related liver disease (ALD) is a major problem worldwide and remains the most common indication for LT in the United Kingdom.4,5 In contrary, hepatitis C was the most common indication for LT in the United States until 2014 but showed a steady decline in the recent years.6,7 There has been a recent increase in patients with nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) along with obesity and metabolic syndrome.8 This aetiological heterogeneity may influence the indications and outcomes of LT.
The practice of LT is unique in Asia, with an asymmetrical growth of living donor LT (LDLT). This procedure is well embraced across Asian countries because of a multitude of factors, which include a higher liver disease burden, lack of an organised system for identification and distribution of deceased donor organs, cultural and religious barriers to the widespread acceptance of brainstem death and deceased donation and the presence of individual surgical practices.9,10 With a greater availability of deceased donor organs, the demand and hence the number of LDLT in the West has traditionally been lower than in the East.11, 12, 13 HBV-related ESLD is the leading indication for LT in this part of the world, exception being Japan where it is predominantly primary biliary cholangitis followed by HCV.14 Interestingly, there has been a change in the epidemiology of patients with CLD. The introduction of potent antiviral therapy, in particular, direct-acting antiviral (DAA) drugs, has altered the course of chronic HCV-infected patients. Amongst the noncommunicable disease, NAFLD has emerged as the most common cause of CLD. Currently, 24–30% of the general population is estimated to have NAFLD. In 20% of patients with NAFLD, the disease progresses to NASH and in some patients to cirrhosis over a period of 10–15 years.8 This dynamic evolution in the epidemiology of CLD may influence patients undergoing LT.
We aimed to study the impact of this changing epidemiology in patients with ESLD who underwent LT over a 10-year period and re-evaluate the relevance of this paradigm shift in the practice of LT.
Methodology
A retrospective, observational study on all adult patients who underwent LT in our unit from November 2009 to December 2019 was carried out following Institute's internal ethical committee approval. Patients transplanted for acute liver failure, paediatric population, retransplantation and combined liver-kidney transplantation were excluded. In India, DAA therapy for HCV was introduced in 2014; hence, the study population was stratified into two time categories: 2009–2014 (Period 1) and 2015–2019 (Period 2). Demographics, type of transplant (LDLT or deceased donor LT [DDLT]), disease aetiology and comorbidities were analysed between the two periods. The diagnosis of NASH was based on the availability of clinical information, including any of previous records, radiological modalities or presence of metabolic risk factors such as diabetes mellitus (DM) or obesity in the absence of excessive alcohol consumption (>20 units per week for men and more than 14 units per week for women). Diagnosis of hepatocellular carcinoma (HCC) was based on abdominal imaging, such as a triphasic computerised tomography or diffusion-weighted magnetic resonance imaging performed as part of liver transplant evaluation. A subgroup analysis of patients with HCC with respect to their disease aetiology was carried out. Furthermore, preoperative cardiometabolic risk profile such as body mass index (BMI), DM, dyslipidaemia and coronary artery disease (CAD) of the study population was analysed. Patients with increased risk for CAD underwent coronary angiogram as the modality of investigation. These patients were further stratified into high-risk CAD (left anterior descending [LAD] > 70% block, double vessel disease, triple vessel disease and history of percutaneous coronary intervention or coronary artery bypass graft) and low-risk CAD (LAD <70% block, single vessel disease). High-risk CAD patients underwent coronary artery intervention before LT. Posttransplant 1-,3-, and 5-year posttransplant patient survival according to the disease aetiology was calculated.
Statistical analysis
Data were analysed using SPSS v 21.0. Mean, standard deviation, frequency and percentage of variables were calculated. Associations between variables were determined using Fisher's exact test. For continuous variables, median differences were examined by Mann–Whitney U test. Cox regression analysis was used to explore the effect of several variables on the time to a specified event occurred, provided that the assumptions of Cox regression were met. A predictive model was built to assess better estimates of survival probabilities and cumulative hazard. Significant risk factors from the univariate analysis were identified and incorporated into multivariate Cox regression analysis after considering confounding variables. The survival function and the regression coefficients for the predictors were estimated from the observed subjects. The Kaplan–Meier method was used to estimate patient survival of our study cohort. The log rank (Mantel–Cox) test was used to calculate survival between the study groups. The results were considered statistically significant when the P value was <0.05.
Results
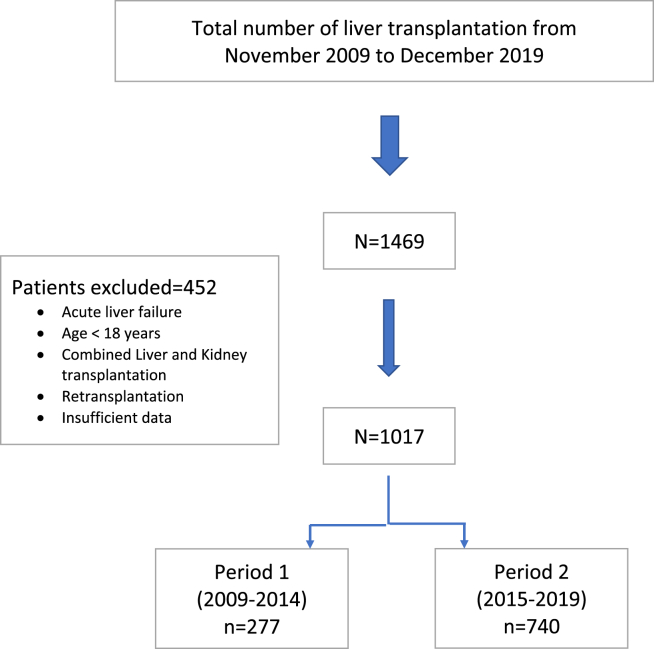
A total of 1469 patients underwent LT between November 2009 and December 2019. After exclusion, we identified 1017 adult patients, of whom 277 in Period 1 (2009–2014) and 740 in Period 2 (2015–2019; Figure 1).
Figure 1.
Flowchart illustrating selection of study population.
Patient demographics are listed in Table 1. There was no statistical difference in the sex ratio (M:F, 4.9:1 vs 3.8:1; P = 0.185), BMI (25.8 vs 25.7 kg/m2; P = 0.971), type 2 DM (96 [34.7%] vs 268 [39.2%]; P = 0.187), hypertension (53 [19.1%] vs 141 [20.6%]; P = 0.658) and CAD (37 [13.4%] vs 77 [11.3%], P = 0.379); whereas age (median 51 [interquartile range (IQR) 43–57] vs 53 [IQR 44–59] years; P = 0.018), model for end-stage liver disease (MELD) score (median, 15 [IQR 11–20] vs 17 [13–22]; P = 0.005), LDLT (183 [66.1%] vs 578 [78%], P = 0.000), and DDLT (94 [33.9%] vs 162 [22%], P = 0.000) were significantly different between Period 1 and Period 2, respectively.
Table 1.
Comparison of characteristics Between Period 1 (2009–2014) and Period 2 (2015–2019).
| Variables | Period 1 (2009–2014) (n = 277) |
Period 2 (2015–2019) (n = 740) |
P value |
|---|---|---|---|
| Sex ratio (M:F) | 4.8:1 | 3.7:1 | 0.185 |
| Age (years), median (IQR) | 51 (43–57) | 53 (44–59) | 0.018 |
| MELD, median (IQR) | 15 (11–20) | 17 (13–22) | 0.005 |
| BMI, median (IQR) | 25.76 (23.24–28.60) | 25.68 (23.05–28.94) | 0.971 |
| LDLT, n (%) | 183 (66.1%) | 578 (78%) | 0.000 |
| DDLT, n (%) | 94 (33.9%) | 162 (22%) | |
| Diabetes mellitus, n (%) | 96 (34.7%) | 268 (39.2%) | 0.187 |
| Systemic hypertension, n (%) | 53 (19.1%) | 141 (20.6%) | 0.658 |
| Coronary artery disease, n (%) | 37 (13.4%) | 77 (11.3%) | 0.379 |
MELD, model for end-stage liver disease; DDLT, deceased donor liver transplantation; LDLT, living (related) donor liver transplantation.
Indications for Liver Transplantation
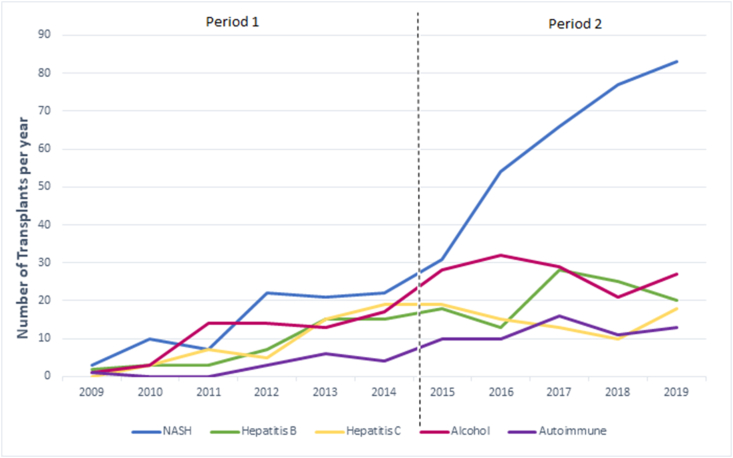
Analysis of indications for LT between two study periods are shown in Table 2. There was a statistically significant increase in patients with NASH (85 [30.7%] vs 311 [42%]; P = 0.001) and a decrease in patients with hepatitis C (49 [17.7%] vs 75 [10.1%]; P = 0.002) from Period 1 to Period 2, respectively. There was no difference in patients with hepatitis B (45 [16.2%] vs 104 [14.1%]; P = 0.372), ALD (62 [22.4%] and 137 [18.5%]; P = 0.183), autoimmune liver disease (14 [5.1%] and 60 [8.1%]; P = 0.104) and HCC patients (52 [18.8%] and 147 [19.9%]; P = 0.723) between during the same study period, respectively. Figure 2 illustrates the changes in disease epidemiology in our study cohort from 2009 to 2019. A subgroup analysis in patients who underwent LDLT (n = 761) between Period 1 (n = 183) and Period 2 (n = 578) also showed an increase in NASH (49 [26.8%] to 242 [41.9%; P < 0.01]) and a decrease in hepatitis C (38 [20.8%] and 61 [10.6%; P = 0.001)), respectively.
Table 2.
Comparative Analysis of Indications for Liver Transplantation Between Two Study Periods.
| Disease aetiology | Period 1 (n = 277) (%) |
Period 2 (n = 740) (%) |
P value |
|---|---|---|---|
| NASH | 85 (30.7%) | 311 (42%) | 0.001 |
| Hepatitis B | 45 (16.2%) | 104 (14.1%) | 0.550 |
| Hepatitis C | 49 (17.7%) | 75 (10.1%) | 0.002 |
| Alcohol | 62 (22.4%) | 137 (18.5%) | 0.183 |
| Autoimmunea | 14 (5.1%) | 60 (8.1%) | 0.104 |
| Othersb | 22 (7.9%) | 53 (7.2%) | 0.089 |
| HCC | 52 (18.8%) | 147 (19.9%) | 0.723 |
NASH, nonalcoholic steatohepatitis; HCC, hepatocellular carcinoma.
Autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis and overlap syndrome.
Drug-induced liver injury, Budd Chiari syndrome, failed Kasai, and Wilson’s disease.
Figure 2.
Liver Transplantation between Period 1 (2009-2014) and Period 2 (2015-2019) based on disease etiology.
Hepatocellular carcinoma
We evaluated the cause of liver disease in HCC patients who underwent LT (n = 199), with 52 (18.8%) and 147 (19.9%) in Period 1 and Period 2, respectively. Demographics showed HCC patients were older (median, 57 [IQR, 52–63] vs 51 [IQR, 42–57] years; P = 0.000), had lower MELD (median, 11 [IQR 8–15] vs 17 [13–22]; P = 0.000), and a trend toward type 2 DM (83 [43.7%] vs 281 [36.5%]; P = 0.079) in comparison with non-HCC patients. Analysis of disease aetiology showed an increase in HCC from Period 1 to Period 2 (13 [26.5%] to 38 [50.7%]; P = 0.009) among HCV patients who underwent LT, whereas no difference in NASH (18 [21.2%] vs 62 [19.9%]; P = 0.879), hepatitis B (16 [35.6%] vs 37 [35.6%]; P = 1.0) and ALD (5 [9.6%] vs 8[5.4%]; P = 0.548) related HCC between the two periods, respectively (Table 3).
Table 3.
HCC Trend Based on the etiology of Liver Disease Between Period 1 and Period 2.
| HCC | Period 1 | Period 2 | P value |
|---|---|---|---|
| NASH | |||
| n = 85 (%) | n = 311, (%) | ||
| NASH HCC | 18 (21.2) | 62 (19.9) | 0.879 |
| Hepatitis B | |||
| n = 45 (%) | n = 104, (%) | ||
| Hepatitis B HCC | 16 (35.6) | 37 (35.6) | 1.0 |
| Hepatitis C | |||
| n = 49 (%) | n = 75 (%) | ||
| Hepatitis C HCC | 13 (26.5) | 38 (50.7) | 0.009 |
HCC: hepatocellular carcinoma; NASH: nonalcoholic steatohepatitis.
Cardiometabolic risk profile in NASH
Preoperative cardiometabolic risk profile of patients who underwent LT from 2009 to 2019 with complete cardiac data set (n = 960) was evaluated. Patients were classified into NASH (n = 372; 38.8%) and non-NASH (n = 588; 61.2%) group. Univariate analysis of risk factors associated with NASH in these patients is illustrated in Table 4. There was a significant difference in age (median 56 [IQR 49.75–61] vs 49 [IQR 40–56] years; P = 0.000), patients with type 2 DM (203 [54.6%] vs 161 [27.4%]; P = 0.000), hypertension (93 [25%] vs 101 [17.2%]; P = 0.004), history of smoking (50 [14%] and 149 [25.9%]; P = 0.000), CAD (65 [17.5%] vs 49 [8.3%]; P = 0.000), MELD (median 16 [12–20] vs 17 [12–22]; P = 0.053), and HbA1c (5.2 [5.0–6.9] vs 5 [4.5–6.0]; P = 0.000), between NASH and non-NASH patients, respectively. A Cox regression analysis of these factors found a strong association between CAD and NASH (hazard ratio [HR] = 1.963, 95% confidence interval [CI] 1.19–3.32; P = 0.00). A subgroup analysis of patients who underwent LDLT (n = 761) also showed a higher incidence of CAD in NASH compared with non-NASH patients (48 [16.5%] vs 35 [7.4%]; P = 0.000), respectively.
Table 4.
Univariate Analysis of Variables Between NASH and Non-NASH Patients.
| Risk factors | NASH (n = 372), median (IQR) |
Non-NASH (n = 588), median (IQR) |
P value |
|---|---|---|---|
| Age (years) |
56 (49.75–61) | 49 (40–56) | 0.000 |
| M:F | 4.3:1 | 3.6:1 | 0.254 |
| BMI | 26.08 (23.53–29.46) | 25.52 (22.84–28.65) | 0.084 |
| MELD | 16 (12–20) | 17 (12–22) | 0.053 |
| HbA1c | 5.2 (5–6.9) | 5 (5–6) | 0.000 |
| Total cholesterol (mg/dL) |
110 (82–135) | 104 (69–133.25) | 0.050 |
| Triglycerides (mg/dL) | 69 (52–89) | 68 (52–92) | 0.947 |
| HDL(mg/dL) | 32 (19–43) | 26 (14–38) | 0.00 |
| Type 2 diabetes mellitus | 203 (54.6%) | 161 (27.4%) | 0.000 |
| Systemic hypertension | 93 (25%) | 101 (17.2%) | 0.004 |
| Coronary artery disease | 65 (17.5%) | 49 (8.3%) | 0.000 |
| Hypothyroidism | 41 (11.1%) | 41 (7%) | 0.032 |
| Alcohol | 65 (18.3%) | 223 (38.8%) | 0.000 |
| Smoking | 50 (14%) | 149 (25.9%) | 0.000 |
BMI: body mass index; IQR: interquartile range; MELD: model for end-stage liver disease; NASH: nonalcoholic steatohepatitis.
CAD: NASH vs non-NASH
Overall, CAD was observed in 114 (11.9%) patients, of whom 65 (57%) had NASH. These patients were further stratified CAD into high-risk (n = 51) and low-risk CAD (n = 63). High-risk CAD patients underwent percutaneous balloon angioplasty along with coronary artery stent insertion before LT. Patients with high-risk CAD were significantly more in the NASH than non-NASH group (27 [7.3%] vs 24 [4.1%]; P = 0.000).
Survival analysis
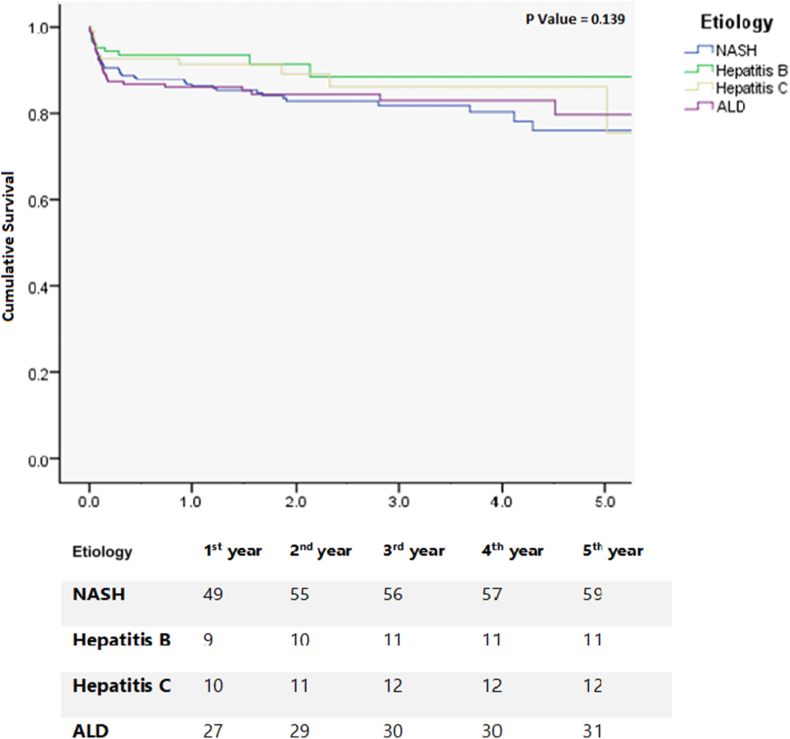
Finally, 1-,3- and 5-year patient survival of our study population showed NASH 86.6%, 81.8% and 75.9%; hepatitis B 93.5%, 88.5% and 88.5%; hepatitis C 91.3%, 86.1% and 86.1%; and ALD 86.0%, 82.9% and 79.7% (P = 0.139), respectively, as illustrated in the Kaplan–Meier curves (Figure 3).
Figure 3.
Kaplan-Meyer survival curves: 1-, 3- and 5-year survival after Liver transplantation based on their disease indications.
Patients transplanted for NASH had a lower 5-year survival compared with those transplanted for viral hepatitis (75.9% vs 87.4%, P = 0.03), with an HR of 1.67 and 95% CI of 1.04–2.69 compared with non-NASH patients. Furthermore, year-on-year 1-year survival since the start of our liver transplant program showed a significantly improved survival from 75% to 91.9% (P = 0.001) from 2009 to 2019. First-year survival based on disease aetiology showed viral hepatitis (85.7%–100%; P = 0.091), NASH (62%–86.9%; P = 0.002), and ALD (75%–96.3%; P = 0.647) from 2009 to 2019, respectively. A univariate analysis showed sepsis (HR 2.3, 95% CI 1.3–4.1; P = 0.004) and graft dysfunction (HR 5.3, 95% CI 1.7–16.2; P = 0.003) for lower survival in patients transplanted for NASH. A multivariate logistic regression analysis identified sepsis (HR 2.8, 95% CI 1.09–7.58; P = 0.031) as the significant risk factor associated with lower survival in NASH patients who underwent LT. Interestingly, the majority of septic complications were observed in the early postoperative period.
Discussion
Evolving indications for LT
NAFLD emerged as the most common CLD causing significant healthcare burden worldwide. Despite effective antiviral therapies, viral hepatitis continues to be a major cause of CLD. This study based on the data from our Institute of Liver disease and transplantation in Chennai, India, clearly demonstrates a substantial increase in NASH over the last 10 years in patients with ESLD undergoing LT. Dynamic alterations in the epidemiology of CLD may have a similar propensity in patients with ESLD. NASH as an indication for LT was first recognised in the last decade. In an earlier study from Japan, NASH represented 1.6% of patients undergoing an LT.15 A longitudinal study by Charlton et al. identified NASH as the third leading indication for LT after hepatitis C and ALD. Interestingly, there was a substantial increase in NASH from 1.2% to 9.7% between 2001 and 2009.16 Similarly, the United Network for Organ Sharing (UNOS) database study of 127,164 patients between 2004 and 2016 revealed significant changes in disease trends in patients with decompensated cirrhosis undergoing LT. With hepatitis C and NASH showing 68% reduction and 97% increment, NASH emerged as the second leading indication for LT. Importantly, NASH was higher among women and Asian patients undergoing LT.17,18 In patients waitlisted for LT from the Scientific Registry of Transplant Recipients (SRTR) database (1994–2016), there was a notable increase in the number of NASH patients (8.3%–19.5%) from 2002 to 2016.19 Intriguingly, even with this increase, O'Leary et al. showed that NASH patients were less likely to receive LT, probably alluding to their age of presentation, presence of obesity and other comorbidities.20 In a more recently published European Liver Transplant Registry (ELTR) database, despite the increase in NASH from 1.2% to 8.4% from 2002 to 2016, ALD remained the most common indication for LT.21 Our study clearly demonstrates NASH (42%) as the most common indication for LT, particularly from the year 2015.
Studies from India showed a 16.6%–24.9% prevalence of NAFLD based on abdominal ultrasound.22,23 A large single-centre epidemiological study from India on 4331 hospitalised cirrhotic patients from 2005 to 2017 identified alcohol (63.3%, n = 2742) as the most common cause of cirrhosis followed by viral hepatitis (19.8%, n = 858) and NAFLD (16.9%, n = 731). From 2005 to 2017, there was a decline in patients with viral hepatitis (39.4%–14.6%), without much changes in the NAFLD group (23.6%–23.3%).24 Although our study shows a decline in HCV, there was a substantial increase in NASH in patients for LT. A small study from our Tamil Nadu state in South India revealed ALD followed by chronic hepatitis B infection but not NASH, as common causes of CLD.25
In India, DAA therapy became available in 2014; hence, we chose 2009–2014 as Period 1 and subsequent years as Period 2 for this study purpose. Our study shows a reduction in patients transplanted for chronic HCV between Period 1 and Period 2 (17.1% vs 10.1%, P = 0.002) in correlation with the advent of DAA for the treatment of chronic HCV infection in India. These drugs have made a considerable paradigm shift in the management of chronic hepatitis C infection. Introduction of protease inhibitors, later polymerase inhibitors and subsequent pangenotypic polymerase inhibitors have increased sustained virological response (SVR) significantly to over 95% with an excellent safety profile.26, 27, 28, 29, 30 SVR has consistently improved several aspects of HCV-related complications. Achieving SVR has strongly shown to reduce the chance of liver disease progression with reduction in portal pressure and death by 74%.31,32 Several studies showed a significant improvement in MELD score, leading to delisting of patients waitlisted for LT. In a study involving 409 patients with HCV ESLD, the mean MELD score decreased by 0.85 within 6 months (P < 0.0001) with reduced episodes of hepatic decompensation (3.7% vs 10%, P = 0.009) in patients who achieved SVR compared with untreated patients.33 Importantly, a cohort study from SRTR database found a 32% reduction in the HCV LT waitlist after achieving SVR, explaining the key role of DAA therapy in these patients.34 Our results concurs with these studies, but in addition, we demonstrate a significant increase in patients with NASH undergoing LT from 2015 onwards.
HCC
In HCV patients undergoing LT, we observed a relative but significant increase in HCC from Period 1 to Period 2 (26.5%–50.7%, P = 0.009). In comparison, there was no difference in NASH-related HCC between the study period (21.2% vs 19.9%; P = 0.879). Chronic hepatitis C is the leading cause of HCC, with 2–8% annual incidence in patients with cirrhosis. HCV-related HCC is the leading indication for LT in the West.35 Controversies exist between risk of HCC and SVR. Achieving SVR has been shown to reduce HCC from 17.8% to 4.2% in the peg-Interferon era.36 Unfortunately, initial reports showed a higher incidence of HCC after achieving SVR after DAA therapy. Conti et al. followed HCV cirrhotic patients treated with DAA therapy for 24 weeks and detected new HCC in 7.6% of patients, which was higher than the annual incidence of HCC in HCV untreated patients.37 Incidence of HCC in HCV cirrhotics after SVR was reported between 3.16 and 9.1%.38 In addition, the incidence of HCC recurrence after resection or ablation was 25–30% higher in HCV-treated patients.39 Similar explanation could justify the reason behind the increase in HCV-related HCC in Period 2 of our study population. Other possibility is the increased longevity of cirrhotic patients following viral clearance with higher chance of developing HCC. A simpler explanation could be the relative reduction in hepatic decompensation after DAA therapy selecting out HCC in HCV patients. A number of studies refuted the added risk of HCC in HCV patients after DAA therapy. In the largest Veterans Affairs Healthcare System database, follow-up study involving 62,354 chronic hepatitis C treated with a mean follow-up of 6.1 years showed a 71% reduction in HCC after SVR irrespective of the type of antiviral therapy.40 In a 10-year study by Sadler et al., 60 (6.5%) and 522 (60.2%) HCC patients were transplanted for NASH and HCV, respectively.41 HCV-related HCC declined along with reduction in chronic HCV infection. A meta-analysis of 41 studies on HCV patients treated with DAA also confirmed 63% reduction in the development of HCC.42
With increasing global prevalence of metabolic syndrome, NASH-related HCC would be an important health care issue.43 HCC occurs in 2.6% of NASH patients and is increasing worldwide.44 A large population (4046 patients)-based study from 2011 identified NASH (58.5%) as the most common cause of HCC.45 However, this interpretation has not yet translated in patients for LT. It would be interesting to observe the influence of changing epidemiology of CLD impacting HCC in the near future.
Coronary artery disease
Analysis of cardiometabolic risk profile of our study population (2009–2019) showed NASH patients undergoing LT were older (P = 0.000), overweight (P = 0.084), with type 2 DM (P = 0.000), hypertension (P = 0.004) and, importantly, higher CAD (P = 0.000). NASH and CAD may have a strong association between, with 65% increase in cardiac events.46 A meta-analysis showed considerably higher (OR 2.05, P < 0.0001) cardiovascular morbidity and mortality in patients with NAFLD.47 In fact, cardiovascular disease is the most common cause of death in patients with metabolic syndrome, particularly in the younger age group.48,49 A Cox regression analysis of our data showed a strong association between CAD and NASH (HR.1.96, 95% CI 1.19–3.32; P = 0.08). Our data support that NASH patients undergoing LT carry a higher cardiometabolic risk by virtue of their comorbidities.
NAFLD and CAD may not just be a mere association, and it is possible that NASH increases the risk of CAD.50 A systematic review by Sookian et al. showed a strong correlation between carotid artery intimal thickness, cholesterol plaques and NAFLD.51 Carotid artery intimal thickness is a surrogate marker of atherosclerosis and is associated with cardiac events.52 NAFLD has been associated with increased coronary artery calcium score, dysregulated coronary artery function and reduced coronary blood flow.53,54 A large meta-analysis with 164,494 participants showed NAFLD was associated with increased cardiovascular disease (OR 1.81). In addition, the meta-analysis clearly demonstrated fatal (OR 2.58) and nonfatal cardiovascular events in patients with NASH.46 Treating clinicians may encounter such high-risk NASH patients imposing additional risk in the pre- and post-transplant period.
NASH: postliver transplant survival
In this study, we found lower 5-year survival in NASH patients compared with those transplanted for viral hepatitis (75.9% vs 87.4%; P = 0.03; HR 1.67, 95% CI 1.04–2.69). Similar to our study, a recent large ELTR analysis showed 1- and 5-year survival of 84.1% and 73.4% in patients transplanted for NASH cirrhosis,22. Likewise, a large UNOS database analysis showed 1-, 3- and 5- year survival of 87.6%, 82.2% and 76.7%, respectively, in patients transplanted for NASH.55 In contrary to our results, a large US database study from a slightly earlier period (2003–2014) revealed a higher 5-year post-LT survival in NASH compared with HCV (77.8% vs 72.1% P < 0.001).56
Our study clearly shows that NASH is the leading indication for LT in India, surpassing viral hepatitis in recent years. This may be as a result of striking increase in the overall prevalence of obesity and metabolic syndrome coupled with the reduction in chronic hepatitis C infection. An increase in patients with NASH undergoing LT is a major concern because of its association with comorbidities such as obesity, DM and CAD. This is a clear warning that in future, we would encounter these high-risk patients posing challenges to the liver transplant team. Multidisciplinary efforts should be taken at the primary care level to curtail obesity, NAFLD and metabolic syndrome in the society.
Credit authorship contribution statement
D.J. contributed to conceptualisation, data curation, analysis and writing and reviewing the article. S.D. contributed to data curation, formal analysis and visualisation. G.N., I.K., Ak.R., K.P., As.R. and R.R. reviewed and edited the article. S.P. contributed to data curation. H.R. did formal analysis. M.R. contributed to reviewing the article and overall supervision.
Conflicts of interest
The authors have none to declare.
Funding
None.
References
- 1.Trotter J.F. Liver transplantation around the world. Curr Opin Organ Transplant. 2017;22:123–127. doi: 10.1097/MOT.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 2.Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 3.Petruzziello A., Marigliano S., Loquercio G., Cozzolino A., Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824–7840. doi: 10.3748/wjg.v22.i34.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal A.K., Bataller R., Ahn J., Kamath P.S., Shah V.H. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113:175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuberger J. Liver transplantation in the United Kingdom. Liver Transplant. 2016;22:1129–1135. doi: 10.1002/lt.24462. [DOI] [PubMed] [Google Scholar]
- 6.Dultz G., Graubard B.I., Martin P., et al. Liver transplantation for chronic hepatitis C virus infection in the United States 2002-2014: an analysis of the UNOS/OPTN registry. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186898. Published 2017 Oct 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim W.R., Lake J.R., Smith J.M., et al. OPTN/SRTR 2017 annual data report: liver. Am J Transplant. 2019;19(Suppl 2):184–283. doi: 10.1111/ajt.15276. [DOI] [PubMed] [Google Scholar]
- 8.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 9.Rela M., Reddy M.S. Living donor liver transplant (LDLT) is the way forward in Asia. Hepatol Int. 2017;11:148–151. doi: 10.1007/s12072-016-9780-z. [DOI] [PubMed] [Google Scholar]
- 10.Narasimhan G., Kota V., Rela M. Liver transplantation in India. Liver Transplant. 2016;22:1019–1024. doi: 10.1002/lt.24459. [DOI] [PubMed] [Google Scholar]
- 11.Matesanz R., Marazuela R., Coll E., et al. About the opt-out system, live transplantation, and information to the public on organ donation in Spain … Y olé! Am J Transplant. 2017;17:1695–1696. doi: 10.1111/ajt.14296. [DOI] [PubMed] [Google Scholar]
- 12.Rhee J., Kern B., Cooper J., Freeman R.B. Organ donation. Semin Liver Dis. 2009;29:19–39. doi: 10.1055/s-0029-1192053. [DOI] [PubMed] [Google Scholar]
- 13.Miller C.M., Quintini C., Dhawan A., et al. The international liver transplantation society living donor liver transplant recipient guideline. Transplantation. 2017;101:938–944. doi: 10.1097/TP.0000000000001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umeshita K., Eguchi S., Egawa H., et al. Liver transplantation in Japan: registry by the Japanese liver transplantation society. Hepatol Res. 2019;49:964–980. doi: 10.1111/hepr.13364. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T., Sugawara Y., Tamura S., et al. Living donor liver transplantation for non-alcoholic steatohepatitis: a single center experience. Hepatol Res. 2014;44:E3–E10. doi: 10.1111/hepr.12200. [DOI] [PubMed] [Google Scholar]
- 16.Charlton M.R., Burns J.M., Pedersen R.A., Watt K.D., Heimbach J.K., Dierkhising R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 17.Wieland A., Kohli R. Non-alcoholic steatohepatitis as a growing indication for liver transplantation: the evolving gender and ethnic trends. Am J Gastroenterol. 2018;113:1588–1589. doi: 10.1038/s41395-018-0373-4. [DOI] [PubMed] [Google Scholar]
- 18.Noureddin M., Vipani A., Bresee C., et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113:1649–1659. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golabi P., Bush H., Stepanova M., et al. Liver transplantation (LT) for cryptogenic cirrhosis (CC) and nonalcoholic steatohepatitis (NASH) cirrhosis: data from the scientific registry of transplant recipients (SRTR): 1994 to 2016. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000011518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Leary J.G., Landaverde C., Jennings L., Goldstein R.M., Davis G.L. Patients with NASH and cryptogenic cirrhosis are less likely than those with hepatitis C to receive liver transplants. Clin Gastroenterol Hepatol. 2011;9:700–704. doi: 10.1016/j.cgh.2011.04.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halder D., Kern B., Hodson J., et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European Liver Transplant Registry study. J Hepatol. 2019;71:313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S.P., Nayak S., Swain M., et al. Prevalence of nonalcoholic fatty liver disease in coastal Eastern India: a preliminary ultrasonographic survey. Trop Gastroenterol. 2004;25:76–79. [PubMed] [Google Scholar]
- 23.Amarapurkar D., Kamani P., Patel N., et al. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161–163. [PubMed] [Google Scholar]
- 24.Mishra D., Dash K.R., Khatua C., et al. A study on the temporal trends in the etiology of cirrhosis of liver in coastal eastern odisha. Euroasian J Hepatogastroenterol. 2020;10:1–6. doi: 10.5005/jp-journals-10018-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvi C., Aravind A., Kumar K. Clinical profile of patients with chronic liver disease in north west tamilnadu. Indian J Appl Res. 2017 doi: 10.36106/ijar. [DOI] [Google Scholar]
- 26.Kieffer T.L., Sarrazin C., Miller J.S., et al. Telaprevir and pegylated interferon-alpha-2a inhibit wild-type and resistant genotype 1 hepatitis C virus replication in patients. Hepatology. 2007;46:631–639. doi: 10.1002/hep.21781. [DOI] [PubMed] [Google Scholar]
- 27.McHutchison J.G., Everson G.T., Gordon S.C., et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–1838. doi: 10.1056/NEJMoa0806104. [published correction appears in N Engl J Med. 2009 Oct 8;361(15):1516] [DOI] [PubMed] [Google Scholar]
- 28.Jothimani D., Chandy G.M., Conjeevaram H. A new era in the treatment of chronic hepatitis C infection. Indian J Gastroenterol. 2013;32:71–79. doi: 10.1007/s12664-012-0254-5. [DOI] [PubMed] [Google Scholar]
- 29.Ghany M.G., Morgan T.R., AASLD-IDSA Hepatitis C Guidance Panel Hepatitis C guidance 2019 update: American association for the study of liver diseases-infectious diseases society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71:686–721. doi: 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu; European association for the study of the liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Lens S., Alvarado-Tapias E., Mariño Z., et al. Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis. Gastroenterology. 2017;153:1273–1283. doi: 10.1053/j.gastro.2017.07.016. e1. [DOI] [PubMed] [Google Scholar]
- 32.Backus L.I., Belperio P.S., Shahoumian T.A., Mole L.A. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology. 2019;69:487–497. doi: 10.1002/hep.29408. [DOI] [PubMed] [Google Scholar]
- 33.Foster G.R., Irving W.L., Cheung M.C., et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Flemming J.A., Kim W.R., Brosgart C.L., Terrault N.A. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65:804–812. doi: 10.1002/hep.28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prenner S.B., Kulik L. Hepatocellular carcinoma in the wait-listed patient with hepatitis C virus. Curr Opin Organ Transplant. 2018;23:237–243. doi: 10.1097/MOT.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 36.Morgan R.L., Baack B., Smith B.D., Yartel A., Pitasi M., Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 37.Conti F., Buonfiglioli F., Scuteri A., et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–733. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Llovet J.M., Zucman-Rossi J., Pikarsky E., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. Published 2016 Apr 14. [DOI] [PubMed] [Google Scholar]
- 39.Reig M., Mariño Z., Perelló C., et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719–726. doi: 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Ioannou G.N., Green P., Kerr K.F., Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. 2019;71:523–533. doi: 10.1016/j.jhep.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadler E.M., Mehta N., Bhat M., et al. Liver transplantation for NASH-related hepatocellular carcinoma versus non-NASH etiologies of hepatocellular carcinoma. Transplantation. 2018;102:640–647. doi: 10.1097/TP.0000000000002043. [DOI] [PubMed] [Google Scholar]
- 42.Waziry R., Hajarizadeh B., Grebely J., et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204–1212. doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 43.Siegel A.B., Zhu A.X. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ascha M.S., Hanouneh I.A., Lopez R., Tamimi T.A., Feldstein A.F., Zein N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 45.Starley B.Q., Calcagno C.J., Harrison S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 46.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Musso G., Gambino R., Cassader M., Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 48.Hamaguchi M., Kojima T., Takeda N., et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunn W., Xu R., Wingard D.L., et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263–2271. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams L.A., Anstee Q.M., Tilg H., Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 51.Sookoian S., Pirola C.J. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600–607. doi: 10.1016/j.jhep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Lorenz M.W., Markus H.S., Bots M.L., Rosvall M., Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 53.Lautamäki R., Borra R., Iozzo P., et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2006;291:E282–E290. doi: 10.1152/ajpendo.00604.2005. [DOI] [PubMed] [Google Scholar]
- 54.Sung K.C., Jeong W.S., Wild S.H., Byrne C.D. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care. 2012;35:717–722. doi: 10.2337/dc11-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Afzali A., Berry K., Ioannou G.N. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transplant. 2012;18:29–37. doi: 10.1002/lt.22435. [DOI] [PubMed] [Google Scholar]
- 56.Cholankeril G., Wong R.J., Hu M., et al. Liver transplantation for nonalcoholic steatohepatitis in the US: temporal trends and outcomes. Dig Dis Sci. 2017;62:2915–2922. doi: 10.1007/s10620-017-4684-x. [DOI] [PubMed] [Google Scholar]