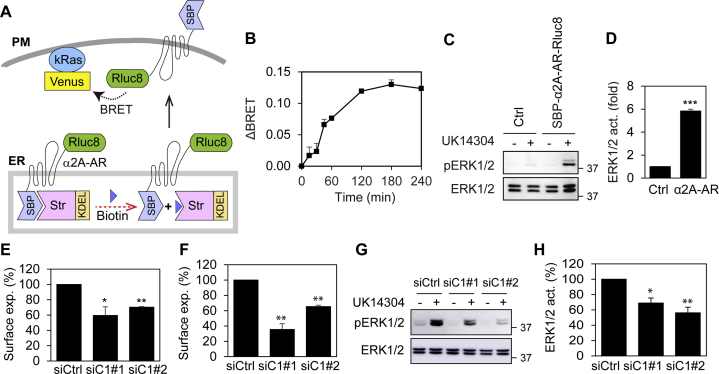
Figure 4.
C1orf27 depletion attenuates the surface transport and signaling of α2A-AR. A, schematic diagram showing modified BRET assays to measure the cell surface transport of nascent α2A-AR after synthesis in the ER and induction with biotin using the RUSH system. B, the surface transport of α2A-AR as measured in live cell RUSH-based BRET assays. HEK293 cells were transfected with Str-KDEL_SBP-α2A-AR-Rluc8 and Venus-kRas for 20 h and then induced with biotin. The surface expression of α2A-AR was measured by BRET assays. C, ERK1/2 activation by Str-KDEL_SBP-α2A-AR-Rluc8. HEK293 cells transfected with Str-KDEL_SBP-α2A-AR-Rluc8 or control plasmids were treated with biotin for 2 h and then stimulated with UK14304 at 1 μM for 5 min. D, quantitative data shown in C. E, inhibition of the surface transport of α2A-AR by C1orf27 siRNA as measured in live cell RUSH-based BRET assays. HEK293 cells were transfected with Str-KDEL_SBP-α2A-AR-Rluc8 and Venus-kRas together with control or C1orf27 siRNA. After incubation with biotin for 2 h, the surface expression of α2A-AR was measured by BRET assays. F, inhibition of the surface expression of α2A-AR by C1orf27 siRNA as measured in ELISA. G, effect of C1orf27 knockdown on α2A-AR–mediated ERK1/2 activation. HEK293 cells were transfected with α2A-AR together with control or C1orf27 siRNA and stimulated with UK14304 at 1 μM for 5 min. H, quantitative data shown in G. The quantitative data are mean ± SE (n = 3). ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 versus control. α2A-AR, α2A-adrenergic receptor; BRET, bioluminescence resonance energy transfer; ER, endoplasmic reticulum; ERK1/2, extracellular signal–regulated kinase 1 and 2; PM, plasma membrane; RUSH, retention using the selective hooks.