Fig. 7.
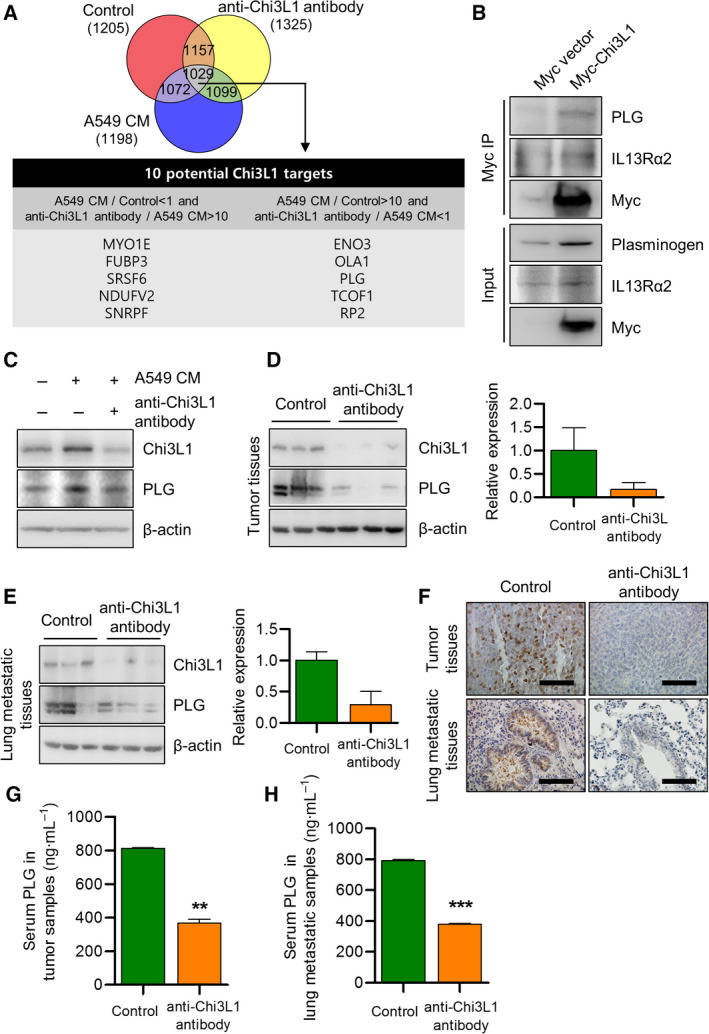
Anti‐Chi3L1 antibody treatment decreases PLG protein production and mRNA expression. (A) THP‐1 was activated using phorbol 12‐myristate 13‐acetate (PMA) and treated with A549 conditioned medium (CM). Processed peptides were subjected to nano‐high‐performance liquid chromatography (nano‐LC) and overlap between the 1029 annotated proteins was identified in THP‐1 (1881), A549 conditioned medium(CM) stimulated THP‐1 (1670, and anti‐Chi3L1 antibody‐treated THP‐1 (1804). (B) A549 cells were transfected with either Myc‐vector or Myc tagged Chi3L1 plasmids. The cell lysates were immunoprecipitated using the anti‐Myc antibody and then subjected to immunoblot analysis with the indicated antibodies. (C) THP‐1 was treated with phorbol 12‐myristate 13‐acetate (PMA) (100 ng·mL−1) and stimulated with A549 conditioned medium (CM) with or without anti‐Chi3L1 antibody (1 μg·mL−1). Western blot was performed to measure the plasminogen (PLG) protein level. (D, E) Tumor tissue and lung metastatic tissue samples were analyzed with immuno‐blotting and (F) immunohistochemistry using the antiplasminogen (PLG) antibody. Data are presented as mean ± standard deviation (SD) from three independent experiments. Scale bar, 100 μm. (G) Serum levels of plasminogen (PLG) in vehicle‐ or anti‐Chi3L1 antibody‐treated tumor tissues. Data are presented as mean ± standard deviation (SD) from three independent experiments. **P < 0.01; (unpaired two‐tailed t‐test). (H) Serum levels of plasminogen (PLG) in vehicle‐ or anti‐Chi3L1 antibody‐treated lung tissues. Data are presented as mean ± standard deviation (SD) from three independent experiments. ***P < 0.001; (unpaired two‐tailed t‐test).
