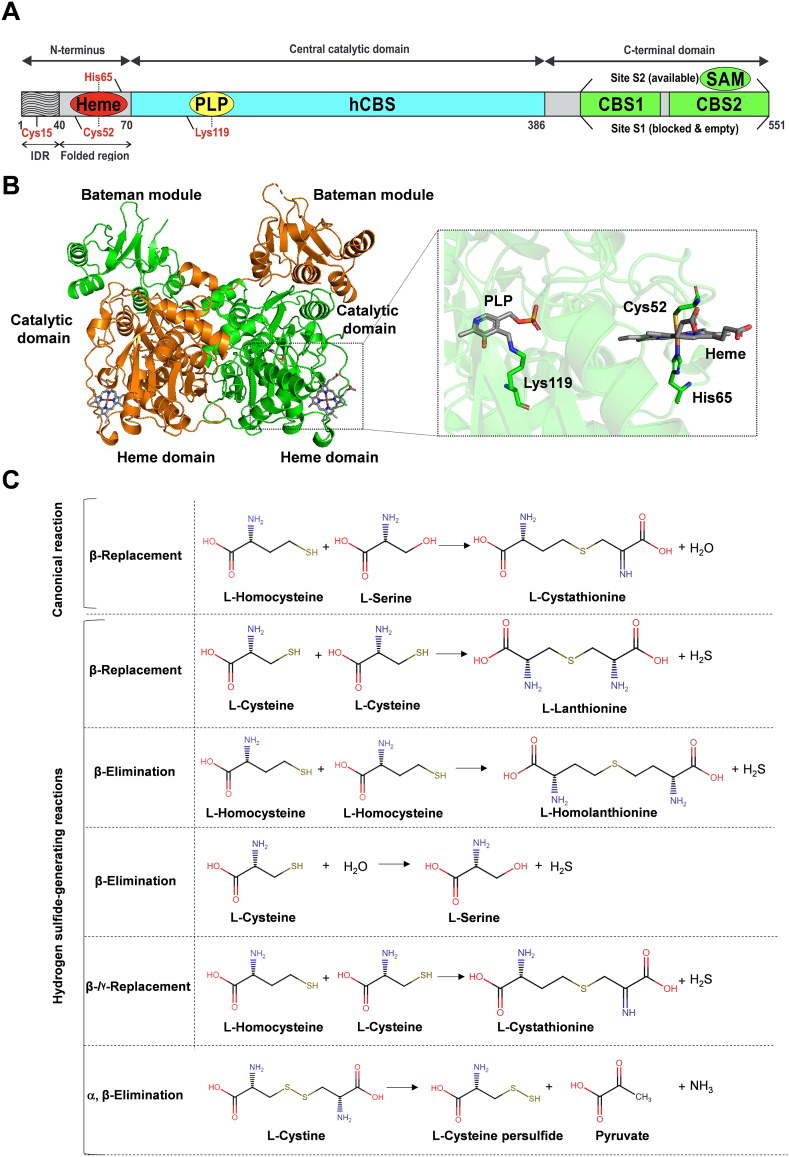
Fig. 1.
Structure and function of CBS. A) The organization of CBS. B) Crystal structure of the Δ516-525 human CBS homodimer (PDB# 4COO). Human CBS is architecturally organized in three regions: the Bateman module, the catalytic domain and the heme-binding domain. The engineered hCBS Δ516-525 is catalytically identical to the full-length native enzyme even if it lacks a loop consisting of 10 amino acid residues from the C-terminal regulatory domain. hCBS Δ516-525 forms dimers, rather then tetramers or higher order oligomers typical of the full-length CBS, that are colored in green and orange, respectively. The PLP and the heme cofactors are shown in sticks. The inset represents a zoom-in view into the catalytic (PLP) and regulatory (heme) sites. The PLP forms an internal aldimine intermediate via the Schiff base bond with the amino group of Lys119, while the heme is coordinated by Cys52 and His65. Figures were generated with PyMol 2.5. C) Key biochemical reactions catalyzed by hCBS. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)