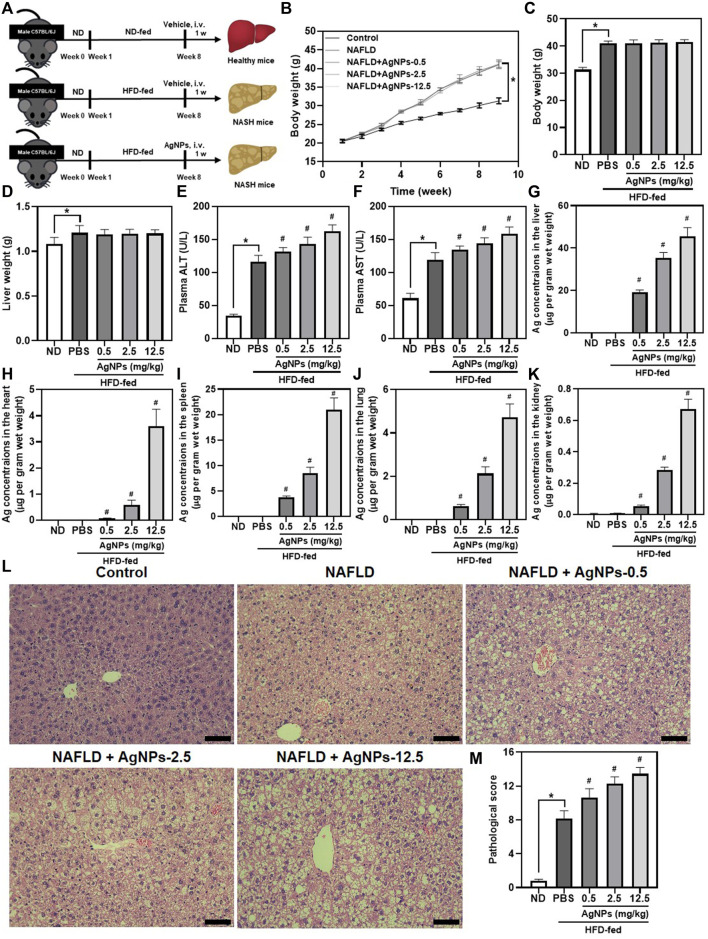
FIGURE 2.
AgNPs aggravated HFD-induced hepatic dysfunction and liver injury in mice. (A) Schematic illustration of the murine model of NAFLD induced by HFD along with AgNP treatment once at the concentration of 0.5, 2.5, and 12.5 mg/kg for 1 week. (B)The daily changes in body weight of the mice during the 8-weeks feeding and 1-week treatment course. The statistical analysis of the body weight (C) and liver weight (D) were obtained from the NAFLD mice after 1-week treatment. (E–F) The liver function of the NAFLD without and with AgNPs was analyzed by the levels of plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST). ICP-MS analysis of the biodistribution of AgNPs at the liver (G), heart (H), spleen (I), lung (J), and kidney (K) of HFD-fed mice after intravenous injection at the concentration of 0.5, 2.5, and 12.5 mg/kg for 1 week. Representative images of H&E staining (L) and statistical analysis of the pathological changes (M) of the liver in ND-fed mice and HFD-fed mice treated with AgNPs. Scale bars, 50 μm *p < 0.05 vs. ND-fed group; # p < 0.05 vs. HFD-treated group.