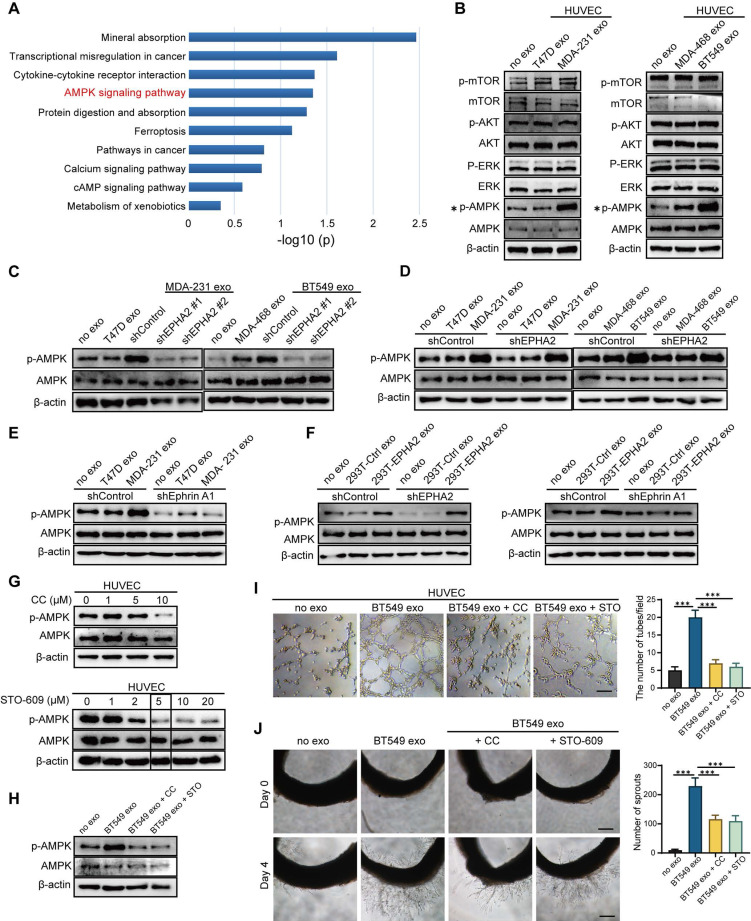
Figure 5.
Exosomal EPHA2 promotes endothelial cell angiogenesis through AMPK signaling pathway. A KEGG enrichment analysis of the RNA-Seq data showed that the AMPK signaling pathway was significantly activated in EPHA2-rich exosomes treated HUVECs compared with control exosome treated cells. B The expression level of p-AMPK was significantly higher in the HM-Exos-treated endothelial cells than in the control and LM-Exos-treated cells, while the phosphorylation levels of Akt, mTOR and ERK were not altered. C Exosomes from EPHA2 stably silenced HM breast cancer cells failed to induce an upregulation of AMPK phosphorylation. D HM-Exos still induced an increase in AMPK phosphorylation in EPHA2-KD HUVECs compared with control cells. E HM-Exos failed to induce an upregulation of p-AMPK in Ephrin A1-KD HUVECs compared with control cells. F Compared with control cells, EPHA2-rich exosomes from HEK-293T cells could induce an increase in AMPK phosphorylation in EPHA2-KD HUVECs, but failed to induce an upregulation of phosphorylated AMPK in Ephrin A-KD HUVECs. G Compound C, an AMPK inhibitor, significantly inhibited AMPK phosphorylation at a concentration of 10 μM; STO609, a CaMKKβ inhibitor, significantly inhibited AMPK phosphorylation at a concentration of 5μM. H Compound C and STO609 eliminated phosphorylation of AMPK in HUVECs after incubation with HM-Exos. I Inhibition of AMPK signaling by Compound C and STO609 reduced tube-forming ability of HUVECs treated with HM-Exos. J Inhibition of AMPK signaling by Compound C and STO609 decreased the ability of microvascular outgrowth in HM-Exos-treated rat arterial rings. All experiments were repeated at least three times. *P < 0.05, **P < 0.01, ***P < 0.001 and ns P > 0.05 indicate no statistical significance. Scale bar: 200 μm.