Abstract
Introduction
Vaccination represents an important strategy to mitigate COVID-19 related morbidity and mortality by protecting against severe forms of the disease and reducing hospitalization and death rates. In this sense, the objective of this study is to estimate the prevalence of Vaccination Intention (VI) against COVID-19 in Latin America and Caribbean (LAC).
Methods
We conducted a systematic review with a comprehensive search strategy for the following databases: PubMed, Scopus and Web of Science. A random-effect model meta-analysis was carried out using observational studies assessing the intention to vaccines against COVID-19 in LAC countries. The Clopper-Pearson method was used to estimate 95% Confidence Intervals. The quality assessment was developed using the Newcastle-Ottawa Scale adapted for cross-sectional studies. A subgroup analysis by study location and a sensitivity analysis were developed.
Results
Nineteen cross-sectional studies were included. Five meta-analyzes were performed according to the target population of the included studies. The VI in the general population of LAC was 78.0% (95%CI: 74.0%–82.0%). The VI for non-pregnant women was 78.0% (95%CI: 58.0%–99.0%), for elderly population was 63.0% (95%CI: 59.0%–69.0%), for pregnant women was 69.0% (95%CI: 61.0%–76.0%) and for health-personnel was 83.0% (95% CI: 71.0%–96.0%). The sensitivity analysis for general population meta-analysis that included only low risk of bias studies showed a 77.0% VI (95%CI: 73.0%–82.0%) and for non-pregnant women, 85.0% VI (95%CI: 79.0%–90.0%).
Conclusion
Despite the high prevalence of VI in general population found in our study, VI prevalence from elderly people and pregnant women are lower than other population groups and overall population.
Keywords: COVID-19, Vaccination intention, Public health
1. Introduction
According to statistics of the World Health Organization, by April 2022, there have been more than 500 million cases of COVID-19 and more than 6 million deaths related to this infection [1]. Regarding Latin America and the Caribbean (LAC), as of April 2022, the number of cases is estimated to be around 72 million and the number of deaths around 1.7 million [1]. Vaccination represents an important strategy to mitigate COVID-19 related morbidity and mortality by protecting against severe forms of the disease and reducing hospitalization and death rates [[2], [3], [4]].
Despite the rapid vaccine development process and implementation of vaccination campaigns against COVID-19, vaccination acceptance continues to be a challenge for health authorities [5]. Previous systematic reviews have found global vaccination acceptance rates ranging from 61 to 73% [6,7]. Also, these reviews highlight the great variability in vaccination acceptance rates depending on the geographic location. For example, in 2021, the acceptance rate of COVID-19 vaccination in Russia was 57.69% [8], while in London was 70% [9].
By June 2021, the COVID-19 vaccination intention rate in LAC was around 80.0% [6]. The highest reported vaccination intention rate was that of Mexico (88.4%), while Venezuela had the lowest one (68.8%) [10]. Some studies have found that the vaccination acceptance also varies depending on the different economic and social factors, such as lower socio-economic level, lower educational attainment and lower age were associated with a lower vaccine uptake [11,12].
The intention to vaccinate also varies depending on the sector in which one works. A study carried out in Colombia found that 90.7% of medical personnel are willing to be vaccinated with an 80.0% effective vaccine [13]. This figure is similar to that reported by studies in Thailand, where a 95.6% vaccination intention was reported by medical personnel [14]. However, a study conducted on Chinese factory workers found that the rate of vaccine acceptance in this population ranged from 66.6% to 80.6% [15].
LAC has been one of the most affected regions by COVID-19 and efforts to continue the vaccination campaigns are needed to reduce the impact of the pandemic in the region. For that reason, information on vaccine acceptance rates and its distribution across different population subgroups is needed. However, currently available reviews that synthetize vaccination acceptance rates mainly include studies from Europe and North America [6,7]. This could be explained by the databases used to conduct the search and the language restrictions when including the individual studies. Thus, the objective of this systematic review is to estimate the prevalence of the intention to vaccinate against COVID-19 in LAC, and to explore how it varies between different age groups. This information could be used to target interventions to promote vaccination.
2. Methods
We conducted a systematic review that adheres to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [16]. A summarized version of the protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) [CRD42021281539].
2.1. Information sources and search strategy
A literature search about acceptance towards COVID-19 vaccination was conducted on September 20, 2021; using controlled vocabulary thesaurus according to each database: PubMed, Scopus and Web of Science (Web of Science Core Collection, SciELO citation index, Russian Science Citation Index and KCI-Korean Journal Database). The search strategy was constructed using the Peer Review of Electronic Search Strategies (PRESS) Checklist [17], with no language restrictions and it is attached as supplemental material (Supplemental Material S1).
2.2. Study selection and data extraction
This systematic review included observational studies (Cohort or Cross-sectional) assessing the acceptance towards COVID-19 Vaccination in adults (≥18 years) living in Latin America and/or the Caribbean. We excluded case reports, scoping reviews, narrative reviews, systematic reviews and conference abstracts. However, we reviewed the secondary studies to search for potential eligible primary studies.
Studies resulting from the bibliographic search were exported to the data management software “Rayyan QCRI” [18]. Four reviewers (FS, VV, EA and JR) assessed independently the titles and abstracts of the retrieved articles. After identifying the potential literature to be included in the review, six reviewers (EA, RU, MM, FS, VV and JR) independently examined the full text of each article using the above mentioned criteria. Discrepancies were discussed to reach a consensus.
The data from the selected articles was collected through a data extraction sheet build in Microsoft Excel. The following information was extracted: title, author, country, year of publication, study design, age, sex, questionnaire administration (face-to-face, online survey), date of survey, response classified as vaccine acceptance, target group, study population, acceptance rate and characteristics of the participants (marital status, socioeconomic status, education and current residence). When data was not available in numerical format, we used the web-based tool “WebPlotDigitizer” to extract the data from graphs [19].
2.3. Evaluation of studies quality and publication bias
To assess the quality of the included studies we used the Newcastle Ottawa scale adapted for Cross-sectional studies (NOS-CS) [20]. A study with 7 or more stars was considered as having a low risk of bias, while a study with less than 7 stars was classified as having a high risk of bias. Four reviewers (EA, RU, FS and VV) independently analyzed the included studies and in case of disagreement over the quality of a study, the team examined the article and reached a consensus.
For this systematic review the team of researchers decided to not carry out the assessment of publication bias due to the fact that conventional funnel plots and egger's tests are inaccurate in proportional meta-analysis. There are two reasons that support the aforementioned statement. In first place, there is no evidence that proportions adjust correctly to these tests. In second place, the tests to assess publication bias were created under the assumption that studies with positive results are published more frequently compared to studies with negative results. However, in this type of study there is no consensus on what a positive result is [21,22].
2.4. Data synthesis and analysis
The information acquired from the included articles was combined using STATA 14.0. We performed a pooled analysis of the acceptance rates with their corresponding 95% confidence intervals (CI). A random effects model (DerSimonian and Laird) was used for the quantitative analysis due to an expected high between-study-heterogeneity. The 95% CI calculations were based on the Clopper-Pearson method. The between-study-heterogeneity was assessed using the Cochran's Q test and the I2 statistic; values greater than 60% were classified as high heterogeneity for the I2 statistic and a P-value <0.05 was a sign of heterogeneity in the Cochran's Q test. Our team performed 5 meta-analyzes in accordance with the target population of the studies: general population, elderly people (>60 years of age), non-pregnant women, pregnant women and health personnel. A sensitivity analysis was carried out eliminating studies with low methodological quality. A subgroup analysis was performed according to the country of origin.
3. Results
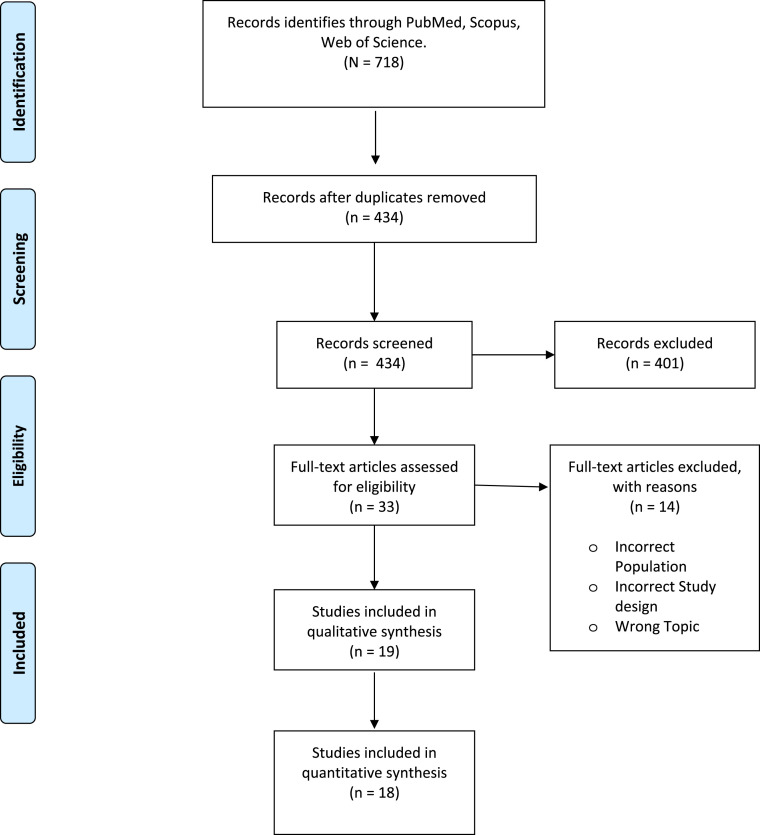
The systematic search yielded 718 articles that were imported to the data management program “Rayyan QCRI”. After the elimination of duplicate studies, we assessed the titles and abstracts of the 434 remaining studies, and 401 articles were eliminated because they did not match the selection criteria. A total of 33 studies were read in full-text and 19 studies were included in the qualitative synthesis and 18 in the quantitative synthesis [10,13,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]]. We did not include the study of Villareal-Garza et al. in the meta-analysis of non-pregnant women, because the study population was specifically women with breast cancer whereas the population of the remaining articles was made up of individuals without a specific pathology. Therefore, including this study in the pooled prevalence could have introduced bias to the results. The selection process is illustrated in the PRISMA flow diagram (Fig. 1 ).
Fig. 1.
PRISMA Flow Diagram for study selection.
3.1. Study characteristics
The 19 studies were all cross-sectional and had a total of 518 941 participants, 62.7% were female and 37.3% males. The geographic distribution was as follows: Brazil (3 articles), Peru (3 articles), Puerto Rico (2 articles), Mexico (2 articles), Colombia (2 articles), Ecuador (1 article), French Guiana (1 article), Chile (1 article), Multiple Countries (4 articles). Most of the questionnaires were administered through an online survey (17 out of 19) and only 2 were administered face-to-face. The dates the surveys were taken ranged from March 2020 to March 2021. The different studies evaluated the acceptance rate in multiple populations; however, these can be summarized in five large groups: general population, elderly people (>60 years old), non-pregnant women, pregnant women and health personnel (belonging to various guilds: medical, nursing, dental, psychology and laboratory personnel). For more information about the characteristics of the studies, see Table 1 .
Table 1.
Characteristics of the included studies.
| Author | Country | Year of publication | Study Design | Target Population | Age (mean ± SD or age ranges and number of participants per range) | Study Population | Sex (N° of Women) | Questionnaire Administration | Date of survey | Response recorded as vaccine acceptance | Acceptance Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ticona JPA | Brazil | 2021 | Cross-sectional | General Population | 39.0 (±14) | 985 | 591 | Face-to-face | November 2020–February 2021 | Yes | 65.9% |
| Vinelli-Arzubiaga D | Peru | 2021 | Cross-sectional | Non- pregnant women | 18–35 years (4753*)/36 or more years (1110*) | 5863 | 5863 | Online Survey | December 2020–January 2021 | Si | 40.0% |
| Caycho-Rodriguez T | Peru | 2021 | Cross-sectional | Elderly Population | 75.3 (±12) | 182 | 87 | Online Survey | February 2021–February 2021 | Muy probable (Very likely)/Bastante probable (Quite likely) | 64.3% |
| Gramacho WG | Brazil | 2021 | Cross-sectional | General Population | 42 | 2771 | 1477 | Online Survey | September 2020–October 2020 | Somewhat likely to take the vaccine/Very likely to take the vaccine | 88.3% |
| Bono SA | Brazil | 2021 | Cross-sectional | General Population | Brazil: 48.0 (±14.6), General: 45.1 (±15.0) | General 10183 | Brazil: 4345, General: 6604 | Online Survey | December 2020–February 2021 | Yes | 95.0% |
| Cerda AA | Chile | 2021 | Cross-sectional | General Population | 18–29: 45*, 30–39: 88*, 40–49: 82*, 50–59: 80*, 60+: 62* | 370 | 216 | Online Survey | August 2020–September 2020 | Yes | 95% Vaccine: 49.0%, 50.0% Vaccine and minor side effects: 36.0%, 95% Vaccine and unknown side effects: 28.0% |
| Skjefte M | Multiple Countries: United States (US), India, Brazil, Russia, Spain, Argentina, Colombia, UK, Mexico, Peru, South Africa, Italy, Chile, Philippines, New Zealand and Australia | 2021 | Cross-sectional | Pregnant women and Non-pregnant women | 34.4 years (±7.3) | 17871 | 17 871 | Online Survey | October 2020–November 2020 | Very likely/Fairly likely/Somewhat likely | Non pregnant mothers: Brazil (92.0%), Mexico (91.0%), Colombia (85.0%), Peru (80.0%), Argentina (79.0%), Chile (79.0%). Pregnant Mothers: Mexico (84.0%), Colombia (72.0%), Brazil (70.0%), Peru (65.0%), Argentina (63.0%), Chile (47.0%) |
| Caycho-Rodriguez T | Peru | 2021 | Cross-sectional | Elderly Population | 72.7 | 245 | 107 | Online Survey | January 2021 | Somewhat likely/Very likely | 65.5% |
| López-Cepero A | Puerto Rico | 2021 | Cross-sectional | General Population | 18-29: 481*, 30–39: 361*, 40–49: 426*, ≥50: 643*. | 1911 | 1444 | Online Survey | December 2020–February 2021 | Yes | 82.5% |
| Alvis-Guzman N | Colombia | 2021 | Cross-sectional | Elderly Population | 80-84: 6469*, 85–89: 3296*, 90–94: 1466*, 95–99: 407*, >100: 83*. | 11721 | 6925 | Online Survey | January 2021–February 2021 | Estoy interesado(a) en ponerme la vacuna/los que querían vacunarse | 60.4% |
| Melin K | Puerto Rico | 2021 | Cross-sectional | General Population | 21-29: 212*, 30–39: 126*, 40–49: 187*, 50–59: 265*, 60+: 226* | 1016 | 767 | Online Survey | July 2020 | Very likely | 69.3% |
| Vignier N | French Guiana | 2021 | Cross-sectional | Health Personnel | 18-34: 187*, 35–49: 198*, 50–64: 152*, 65+: 42* | 579 | 393 | Face-to-face | January 2021–March 2021 | Likely/Done | 64.4% |
| Jaramillo-Monge J | Ecuador | 2021 | Cross-sectional | General Population | Rural: 26.7 (±10.0), suburban: 27.8 (±10.6), urban: 33.5 (±13.3) | 1219 | 693 | Online Survey | February 2021 | They were willing to be vaccinated with a COVID-19 vaccine | 90.9% |
| Stojanovic J | Multiple Countries: Brazil, Canada, Colombia, France, Italy, Turkey, UK, USA. | 2021 | Cross-sectional | General Population | ≤29: 6701*, 30–64: 16005*, ≥65: 3781* | 32028 | 19060 | Online Survey | March 2020–January 2021 | Extremely likely | General: 73.4%, South America: 79.5% |
| Urrunaga-Pastor D | Latin America and the Caribbean | 2021 | Cross-sectional | General Population | <45 years: 331 835* | 472521 | 263 026 | Online Survey | January 2021–February 2021 | Yes, definitely/Yes, probably | 80% |
| Alvarado-Socarras JL | Colombia | 2021 | Cross-sectional | Health Pesonnel | 60% Effectiveness: NO [47.4 (±18)], YES [45.1 (±19)], 80% Effectiveness: NO [48.7 (±20)], YES [45.3 (±19)] | 1066 | 501 | Online Survey | January 2021 | Agree to apply a free vaccine with 60% effectiveness/Accept to apply a free vaccine with 80% effectiveness | 60% Effectiveness: 77.0%, 80% Effectiveness: 90.7% |
| Castañeda-Vasquez DE | Mexico | 2021 | Cross-sectional | Health Personnel | 21 years (18–69**). | 543 | 353 | Online Survey | October 2020–December 2020 | Considers Getting Vaccinated | 94.5% |
| Lazarus JV | Multiple Countries: 19 countries (Brazil, Ecuador, Mexico) | 2020 | Cross-sectional | General Population | Brazil: <50: 545*, ≥50: 172*, Ecuador: <50: 585*, ≥50 : 156*, Mexico: <50: 492* ≥ 50: 203* | 13426 | Brazil: 436, Ecuador: 407, Mexico: 364 | Online Survey | June 2020 | Completely agree/aSomewhat agree | Brazil:85.4%, Ecuador: 71.9%, Mexico: 76.3% |
| Villareal-Garza C | Mexico | 2021 | Cross-sectional | Female patients with breast cancer | 49 (23–85**) | 540 | 540 | Online Survey | March 2021 | Willing to be vaccinated immediately | 66.0% |
3.2. Risk of bias assessment
The quality score of the included studies ranged from 2 to 9 points. A total of 7 studies were classified as being of low quality and 12 of high quality. The section that presented the most shortcomings was that regarding the selection process. An in-depth analysis is presented in Table 2 . In addition to selection bias inherent to observational studies, most of included studies have been conducted through an online survey which can cause selection bias as only those who have internet access and economic resources to acquire mobile phones could be reached. Likewise, people with access to internet are exposed to infodemics, including false or misleading information about COVID-19 vaccines and other disease-related issues.
Table 2.
Quality assessment of the included articles using the Newcastle-Ottawa Scale for Cross-sectional Studies (NOS-CS).
| AUTHOR | SELECTION |
COMPARABILITY | OUTCOME |
OVERALL | ||||
|---|---|---|---|---|---|---|---|---|
| RS | SS | NR | AE | AO | ST | |||
| Ticona JPA | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 |
| Vinelli-Arzubiaga D | ★ | ★ | ★ | ★ | ★ | 5 | ||
| Caycho-Rodriguez T | ★ | ★ | ★ | ★ | 4 | |||
| Gramacho WG | ★ | ★ | ★ | ★ | ★ | ★ | 6 | |
| Bono SA | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 |
| Cerda AA | ★ | ★ | ★ | ★★ | ★ | ★ | 7 | |
| Skjefte M | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | 8 |
| Caycho-Rodriguez T | ★ | ★ | ★ | ★★ | ★★ | ★ | ★ | 9 |
| López-Cepero A | ★ | ★ | ★ | ★★ | ★★ | ★ | ★ | 9 |
| Alvis-Guzman N | ★ | ★ | ★ | ★ | ★ | 5 | ||
| Melin K | ★ | ★ | ★ | ★★ | ★ | ★ | 7 | |
| Vignier N | ★ | ★ | ★ | ★★ | ★ | ★ | 7 | |
| Jaramillo-Monge J | ★ | ★ | ★ | ★★ | ★ | ★ | 7 | |
| Stojanovic J | ★ | ★ | ★★ | ★ | ★ | ★ | 7 | |
| Urrunaga-Pastor D | ★ | ★ | ★ | ★★ | ★ | ★ | 7 | |
| Alvarado-Socarras JL | ★ | ★ | ★ | 3 | ||||
| Castañeda-Vasquez DE | ★★ | ★ | ★ | 4 | ||||
| Lazarus JV | ★ | ★ | ★★ | ★ | ★ | ★ | 7 | |
| Villareal-Garza C | ★ | ★ | 2 | |||||
RS: Representativeness of the sample, SS: Sample Size, NR: Non-respondents, AE: Ascertainment of the exposure, AO: Assessment of the outcome, ST: Statistical Test.
3.3. Pooled estimates of the included studies
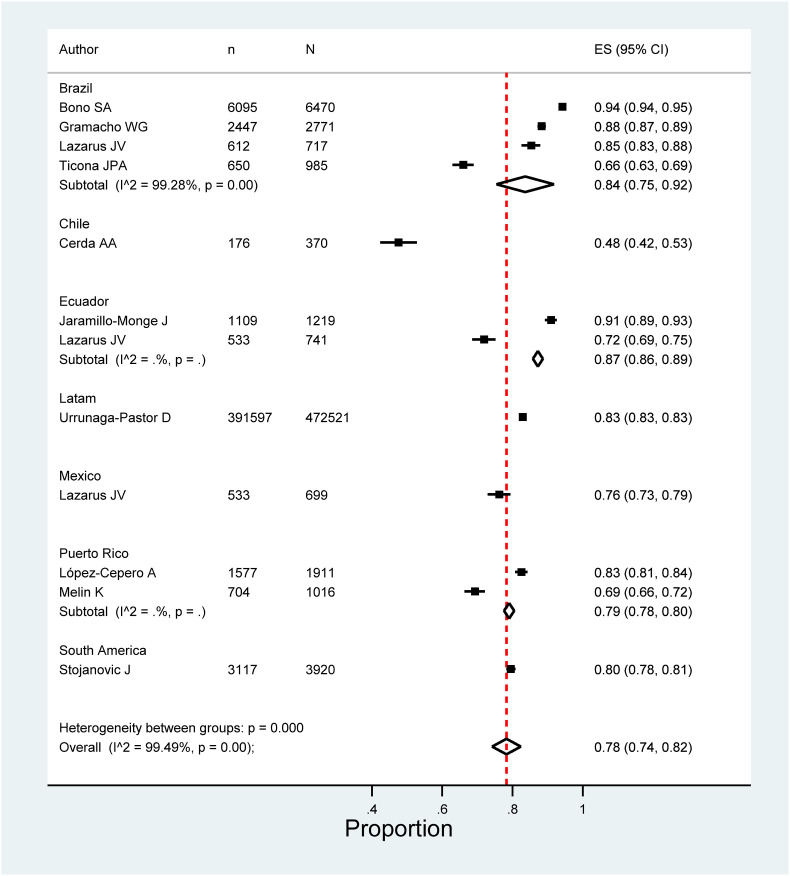
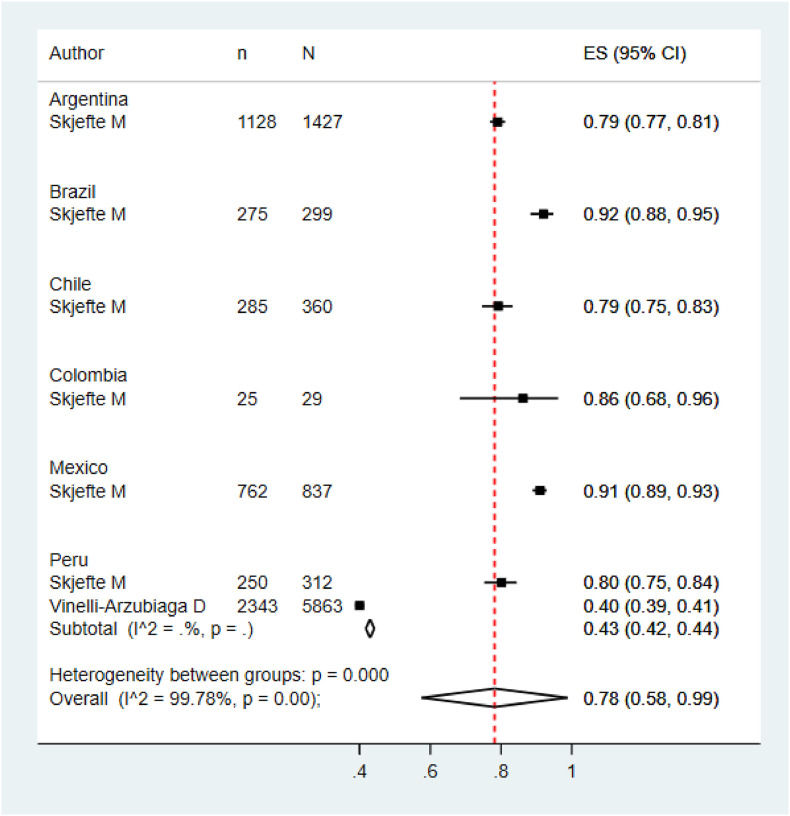
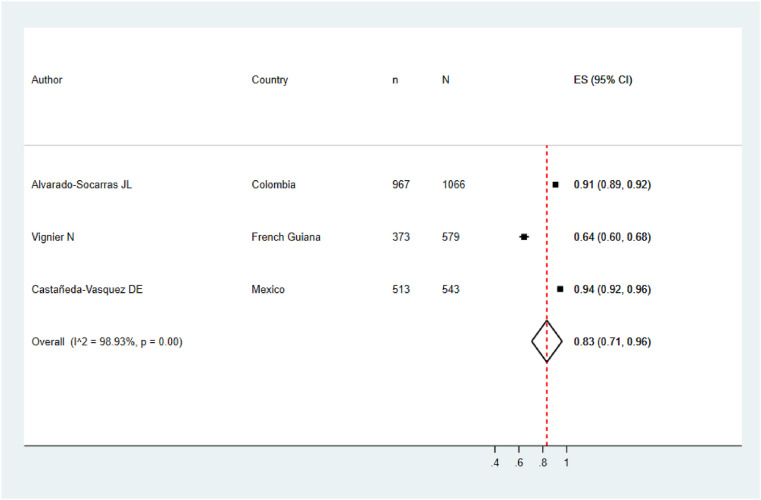
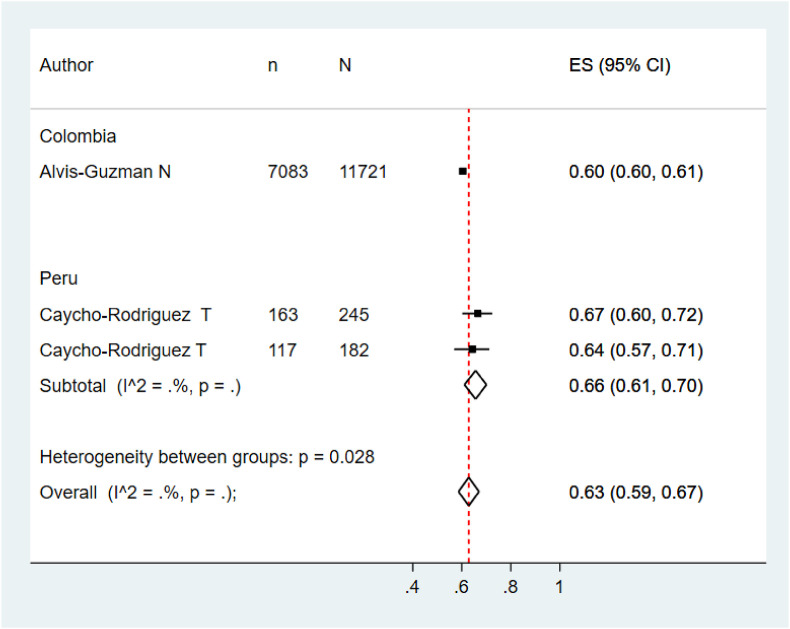
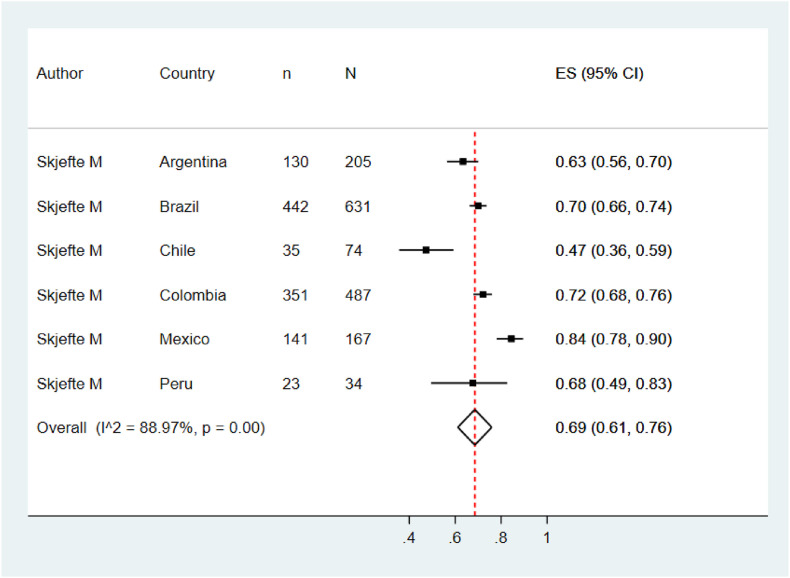
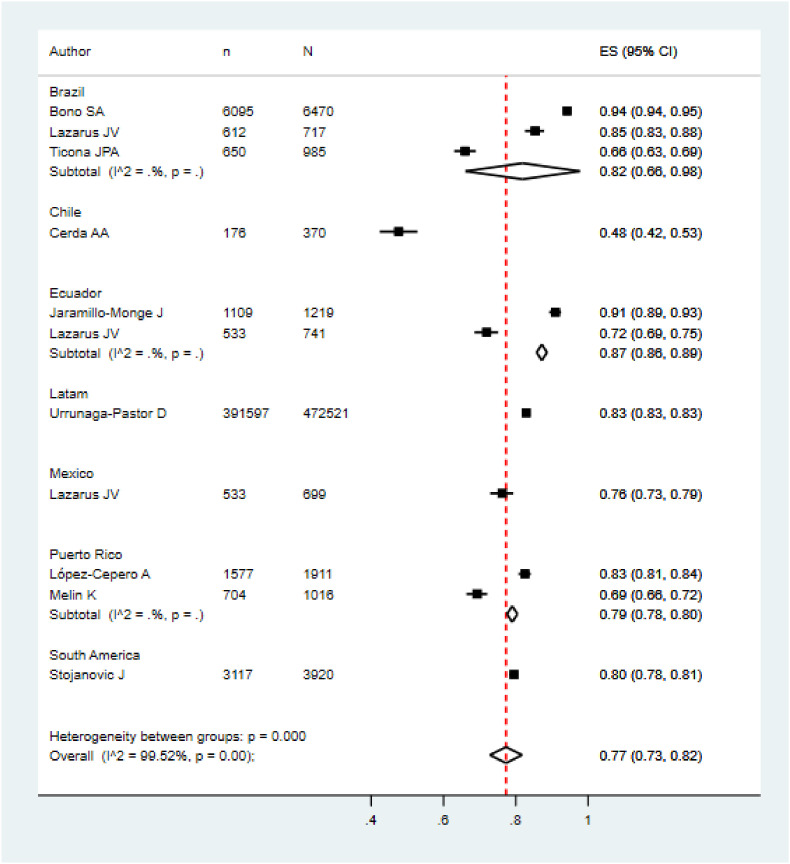
Five meta-analyzes were performed according to the target population of the included studies. The pooled prevalence of COVID vaccination acceptance in the general population was 78.0% (95% CI: 74.0%–82.0%), with significant heterogeneity among studies (See Fig. 2 ). Similar results were found in the pooled analysis of COVID vaccination acceptance rates for non-pregnant women (78.0%, 95% CI: 58.0%–99.0%, I2: 99.8%) and health personnel (83.0%, 95% CI: 71.0%–96.0%, I2: 98.9%) (See Fig. 3, Fig. 4 ). The pooled prevalences of vaccination acceptance in the elderly population and in pregnant women were lower, 63.0% (95% CI: 59.0%–69.0%) and 69.0% (95% CI: 61.0%–76.0%), respectively (See Fig. 5, Fig. 6 ). It is important to highlight that the prevalences of the different Latin American countries reported in the pooled analysis of vaccination acceptances of non-pregnant women came from the same study; this is why the measures of between-study-heterogeneity are not applicable.
Fig. 2.
Prevalence of COVID-19 vaccination intention in the general population.
Fig. 3.
Prevalence of vaccination intention against COVID-19 in non-pregnant women.
Fig. 4.
Prevalence of vaccination intention against COVID-19 in health personnel.
Fig. 5.
Prevalence of vaccination intention against COVID-19 in elderly people.
Fig. 6.
Prevalence of vaccination intention against COVID-19 in pregnant women.
3.4. Subgroup analysis
Regarding to subgroups analysis by country in the general population, countries with more than one study and their pooled VI prevalences were Brazil (84.0%; 95%CI: 75.0%–92.0%), Ecuador (87.0%; 95%CI: 86.0%–89.0%) and Puerto Rico (79.0%; 95%CI: 78.0%–80.0%) (Fig. 2). In elderly people, Peru (66.0%; 95%CI: 61.0%–70.0%) was the only country that accrued at least two studies and the other countries had just one study (Fig. 5). In relation to non-pregnant women, Peru (43.0%; 95%CI: 42.0%–44.0%) had at least two studies and the additional countries had just one study (Fig. 3). In the quantitative analysis of pregnant women and health personnel, there was only one study per country.
3.5. Sensitivity analysis
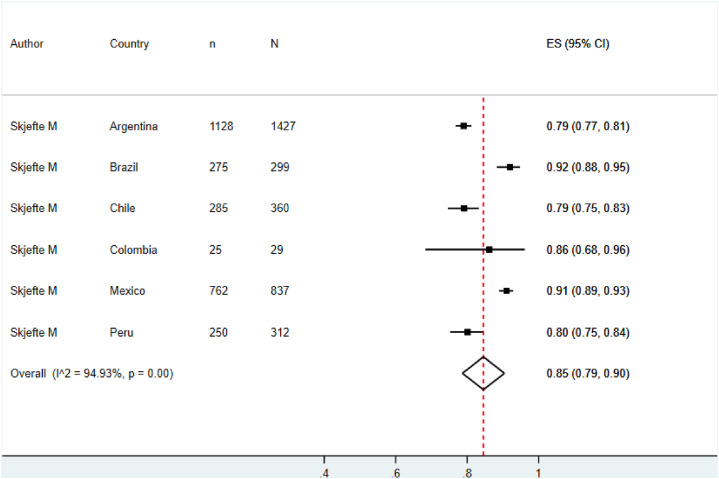
By removing studies with low methodological quality, the pooled prevalence of vaccination acceptance in the general population and its heterogeneity stayed at similar values (77.0%, 95% CI: 73.0%–82.0%, I2: 99.5%) (See Fig. 7 ). In contrast, the pooled prevalence of vaccination acceptance in non-pregnant women rose from 78.0% (95% CI: 58.0%–99.0%) to 85.0% (95% CI: 79.0%–90.0%) (See Fig. 8 ).
Fig. 7.
Sensitivity analysis for prevalence of vaccination intention against COVID-19 in the general population.
Fig. 8.
Sensitivity analysis for prevalence of vaccination intention against COVID-19 in non-pregnant women.
In the rest of the meta-analyzes, it was not possible to perform a sensitivity analysis due to the fact that eliminating studies with a high risk of bias left the model with an insufficient number of studies to give a pooled estimate.
4. Discussion
This systematic review aimed to estimate the prevalence of vaccination intention (VI) in LAC. Our findings showed a high prevalence of VI in the general population of LAC (78.0%) and this proportion was similar for most countries in the meta-analysis. Additionally, we explored the VI in elderly population, pregnant women and non-pregnant women and we found prevalences of 63.0%, 69.0% and 78.0%, respectively.
Up-to-date systematic reviews of VI have not focused on LAC population due to the limited number of primary studies conducted in this region compared to those of North America, Europe and Asia [6,7].Urrunaga et al. [10], used secondary data collected from the University of Maryland and Facebook ® to estimate prevalences of VI for LAC countries. They found prevalences of VI and fear to adverse effects of vaccine of 80.0% and 81.2%, respectively. These results are consistent with other studies that assess COVID-19 vaccine hesitancy worldwide and ours, which show that LAC countries exhibited acceptance rates greater than 70% [40]. These results are encouraging because some countries with the highest numbers of COVID-19 confirmed cases and the highest excess mortality rates belong to the region of LAC [41].
When contrasting with other regions, the prevalence of VI found in our study was relatively higher or similar to that found in some studies conducted in European and Asian countries such as Italy (ranging from 40.1% to 78.5%) [42,43], France (ranging from 30.5% to 77.6%) [44,45], Greece (ranging from 62% to 84.8%) [46,47], Germany (ranging from 58% to 70%) [48,49], UK (ranging from 64% to 73.5%) [50,51], China (ranging from 28.7% to 82%) [52,53], or Bangladesh (ranging from 26% to 65.5%) [54,55]. Likewise, South Africa reported a VI lower than our estimate for LAC [56]. [52] Nevertheless, these prevalences ranged broadly between different studies conducted in the same country or region [57]. These differences could be explained by different factors. The main one would be the time when the studied was carried out, and others factors affecting the VI prevalence would be the target population, trust in the health system, among others.
The high prevalence in LAC could be the result from people's fear to get the severe disease or to die by COVID-19 given the burden of this infectious disease in this region. Several countries in LAC showed high incidence rates of COVID-19 in the first 90-days of the pandemic [58], and some LAC countries, mainly Brazil, Peru and Colombia showed one of the highest excess death rates by COVID-19 in the world [41,59]. In the case of Peru, the country with the highest fatality rate in the world due to COVID-19 and in just 11 months, from February to December 2021, 80% of the population over 12 years completed their immunization process against COVID-19 [60]. LAC accrued almost one-third of COVID-19-related deaths [59]. However, fear of COVID-19 is not the only factor that could affect VI.
Differences between studies could be explained by differences in sociodemographic characteristics, the date when the study was carried out, local contexts, among others. Regarding the VI, differences could be derived from surveys and how intention to vaccinate was asked, for instance some studies asked for VI according to vaccines efficacy [27], prices [61], and other characteristics. Reasons for no acceptance of vaccination against COVID-19 could be fear of adverse effects [62,63], distrust in local health systems [64,65], misinformation or fake news shared in social media [66,67], and other factors for population specific groups. Different factors such as health-system-related variables, local concerns (economy, virtual education, teleworking, etc.), political issues (purchase of vaccine batches, quarantine isolation measures, vaccination process implementation, etc.), demographic and geographical variables could impact VI. Another common factor in the region is the entrenched vaccination culture in LAC population [60], and the promotion of the importance of vaccination at the first level of care despite the limited human resources in health systems. Moreover, the implementation of public policies aimed at promoting vaccination became vitally important. In this sense, the requirement of the vaccination card to get into closed establishments (restaurants, cinemas, etc.) and as a requirement for companies was one of the important strategies of government institutions to promote vaccination in the population at advanced vaccination process stages [68]. Bearing this in mind, governments need to assess what role different factors play when vaccination strategies are developed.
By January 2022, vaccination in LAC is being conducted in all countries and some countries are about to start the vaccination process in children. Almost all LAC countries have already faced their second wave of COVID-19 and a lot of them have modified their strategies or designed new ones to avoid COVID-19 cases from increasing. Nonetheless, vaccination campaigns keep encountering different challenges between LAC countries. In the case of Peru [64,65], political issues and a scandal related to a vaccine clinical trial could have set a distrust from population about vaccine effectiveness, vaccination programs and local health system. Similar problems were reported in Ecuador and Brazil6,7. All these issues have a negative impact on VI of the general population and could constitute a source of refusal to COVID-19 vaccines.
Nowadays, January 2022, vaccination is one of the most efficient public health interventions for preventing severe cases of COVID-19 [69]. However, several factors influence intention of getting vaccinated and could affect decreasing of specific mortality. In turn, this fact would contribute to health care systems collapsing and shortage of health resources. Elderly people were the population with the lowest pooled prevalence of VI (63.0%) despite the fact that older adults are at a higher risk of COVID-19 mortality and morbidity [70]. The VI prevalence from elderly people and pregnant women are lower than other population groups and overall population. This result is a critical concern for public health systems due to people at high risk of COVID-19 severity have less intention to get vaccinated.
On the other hand, few studies reported VI in health personnel from LAC and the pooled prevalence for this group (83.0%) was greater than that of the general population. Probably, it is due to the fact that this group has a better understanding of issues related to COVID-19 and has more knowledge about the vaccination processes. Previous studies in health workers have reported a great knowledge of COVID-19 [71], a low vaccine refusal rate [72,73] and a VI prevalence less than reported by our study [71,73]. In a study conducted in Spain, it was reported that physicians had more confidence in vaccines compared to nurses and their VI was slightly greater than nurses VI [72]. These results are useful to claim that prevalence of VI in health personnel is greater than VI of non-health professionals but it could exist differences of VI prevalence between different professions. Further research may postulate causes for these differences and expand our knowledge about it.
This analysis also showed that the pooled prevalence of VI for pregnant women (69.0%) was slightly greater than that of the elderly population. In previous studies, it was found that female gender was associated with a lower probability of VI [10,40,[74], [75], [76]], and that men have lower risk of accepting conspiracy beliefs about COVID-19 [77,78]. Likewise, a lot of factors were reported as predictors of VI in pregnant women, including confidence in received information about vaccination process and COVID-19 vaccines [28,79,80], belief in the importance of vaccines [28], no fear of vaccine side effects [81], among others. An additional factor we considered is the trust in public health agencies or systems, however, additional research is necessary to establish this association. These reasons could justify differences in prevalence of VI between genders. Another group of interest was non-pregnant women, this group showed the greatest prevalence of VI with 78.0%. In a previous study, several factors, that could explain our result, were associated with a high likelihood of getting vaccinated in non-pregnant women. For instance, a higher odd of VI was associated with an older age, smaller number of children and a higher education degree [28].
The synthetized evidence showed that Ecuador had the highest pooled prevalence of VI (87.0%) followed by Brazil (84.0%) and these high prevalences of VI could derive from high mortality rates in Brazil and Ecuador [82]. The sensitivity analysis was able to be reported just in general population and non-pregnant women because of quality assessment left an insufficient number of studies for the other subgroups. Similar situations were undergone by other LAC countries as Ecuador, where the highest raw mortality rate was registered in the first 90-days of the pandemic [58].
4.1. Strengths and limitations
This study presents some limitations. As studies retrieved from databases are subjected to peer-review process duration and due to COVID-19 pandemic is a current global health emergence, included studies could not reflect the most up-to-date evidence in VI. Our estimates have broad CIs that could be explained by differences between study's methodology. In addition, different vaccination rates, local health systems efficiency, infodemics, and local political concerns, could have introduced differences between studied populations. Likewise, we have tried to estimate a VI for LAC from the available published evidence. Nevertheless, we did not retrieve studies from all LAC countries and most of the included studies were developed with online surveys. In this sense, the VI of people without online access, and who live in countries that were not included in this systematic review may not be represented by our results. Another important limitation is the variability of settings or contexts of included studies between 2020 and 2021. Despite the narrow time period covered by our systematic review, a significant number of different attitudes towards vaccination, factors affecting it, and social contexts may be found in LAC countries. These variables can be important sources of heterogeneity for the systematic review or, on the other hand, prevent a generalization or general representation of all LAC countries.
It is also important to clarify that we assessed VI and not the vaccination rate. The second one is defined as the number of doses applied to population in a specific time interval. This last concept is more objective than VI and it is common to observe differences between VI and vaccination rate for the same country. This exploration is not within the scope of this review, but might include organization of the health care system and accessibility to healthcare centers.
Despite the limitations, this systematic review has important strengths. First, it was registered in the PROSPERO database and the PRISMA statement was followed for reporting our results. Second, we developed a comprehensive search strategy through multiple databases, with no restrictions. Third, a sensitivity analysis was performed including only high-quality studies. Fourth, we approached a relevant issue in this pandemic. These strengths assign a great value to the results of our systematic review and allow contrasting the VI of LAC population and the progress of the vaccination process.
Identifying subgroups with low prevalence of VI could help governments to design more efficient public health strategies about vaccination. In this sense, our study helped to identify population subgroups being less likely to get vaccinated and consequently having a higher risk of getting sick by COVID-19. Our study results constitute an input to evaluate implemented measures about vaccination on LAC countries and could be used as a guide for developing new public health policies focused on population subgroups with low prevalence of VI. Additionally, these results could be used as reference in future pandemics to stratify groups with low VI and build specific strategies for them. Further research is needed to study factors associated to these groups with low VI.
5. Conclusion
Despite the high prevalence of VI in general population found in our study, VI prevalence from elderly people and pregnant women are lower than other population groups and overall population. These results could be used by governments for designing, developing or promoting vaccination strategies and public health policies focused on these populations. Vaccination remains as the most efficient public health intervention for preventing severe cases of COVID-19 and it helps to reduce COVID-19 impact to health systems.
Authors contributions
Esteban A. Alarcón-Braga: Conceptualization, Methodology, Investigation, Formal analysis and Writing - Original Draft. Enrique A. Hernandez-Bustamante: Methodology, Investigation, Formal analysis and Writing - Original Draft. Farley E. Salazar-Valdivia: Methodology, Investigation and Writing - Original Draft. Valeria A. Valdez-Cornejo: Methodology, Investigation and Writing - Original Draft. Melany D. Mosquera-Rojas: Methodology, Investigation and Writing - Original Draft. Juan R. Ulloque-Badaracco: Methodology, Investigation and Writing - Original Draft. Jenny C. Rondon-Saldaña: Methodology, Investigation and Writing - Original Draft. Jessica H. Zafra-Tanaka: Conceptualization, Methodology, Writing - Review & Editing, Visualization and Supervision.
Funding
This research study did not receive any funding.
Declaration of competing interest
The authors do not have any conflict of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tmaid.2022.102369.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/table n.d.
- 2.World Health Organization WHO coronavirus (COVID-19) dashboard | WHO coronavirus (COVID-19) dashboard with vaccination data. https://covid19.who.int/ n.d.
- 3.Sullivan M.P., Meyer P.J. Congressional Research Service; 2021. Latin America and the Caribbean: impact of COVID-19. [Google Scholar]
- 4.Benefits of getting a COVID-19 vaccine | CDC. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html n.d.
- 5.Al-Amer R., Maneze D., Everett B., Montayre J., Villarosa A.R., Dwekat E., et al. COVID-19 vaccination intention in the first year of the pandemic: a systematic review. J Clin Nurs. 2022;31:62–86. doi: 10.1111/JOCN.15951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q., Yang L., Jin H., Lin L. Vaccination against COVID-19: a systematic review and meta-analysis of acceptability and its predictors. Prev Med. 2021;150 doi: 10.1016/J.YPMED.2021.106694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norhayati M.N., Che Yusof R., Azman Y.M. Systematic review and meta-analysis of COVID-19 vaccination acceptance. Front Med. 2022;8:3091. doi: 10.3389/FMED.2021.783982/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran V.D., Pak T.v., Gribkova E.I., Galkina G.A., Loskutova E.E., Dorofeeva V.v., et al. Determinants of COVID-19 vaccine acceptance in a high infection-rate country: a cross-sectional study in Russia. Pharm Pract. 2021;19 doi: 10.18549/PHARMPRACT.2021.1.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trent M., Seale H., Chughtai A.A., Salmon D., MacIntyre C.R. Trust in government, intention to vaccinate and COVID-19 vaccine hesitancy: a comparative survey of five large cities in the United States, United Kingdom, and Australia. Vaccine. 2022;40:2498–2505. doi: 10.1016/J.VACCINE.2021.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urrunaga-Pastor D., Bendezu-Quispe G., Herrera-Añazco P., Uyen-Cateriano A., Toro-Huamanchumo C.J., Rodriguez-Morales A.J., et al. Cross-sectional analysis of COVID-19 vaccine intention, perceptions and hesitancy across Latin America and the Caribbean. Trav Med Infect Dis. 2021;41 doi: 10.1016/J.TMAID.2021.102059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soares P., Rocha J.V., Moniz M., Gama A., Laires P.A., Pedro A.R., et al. Factors associated with COVID-19 vaccine hesitancy. Vaccines. 2021;9 doi: 10.3390/VACCINES9030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzman-Holst A., DeAntonio R., Prado-Cohrs D., Juliao P. Barriers to vaccination in Latin America: a systematic literature review. Vaccine. 2020;38:470–481. doi: 10.1016/J.VACCINE.2019.10.088. [DOI] [PubMed] [Google Scholar]
- 13.Alvarado‐socarras J.L., Vesga‐varela A.L., Quintero‐lesmes D.C., Fama‐pereira M.M., Serrano‐diaz N.C., Vasco M., et al. Perception of COVID-19 vaccination amongst physicians in Colombia. Vaccines. 2021;9 doi: 10.3390/VACCINES9030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sirikalyanpaiboon M., Ousirimaneechai K., Phannajit J., Pitisuttithum P., Jantarabenjakul W., Chaiteerakij R., et al. COVID-19 vaccine acceptance, hesitancy, and determinants among physicians in a university-based teaching hospital in Thailand. BMC Infect Dis. 2021;21:1–12. doi: 10.1186/S12879-021-06863-5. 2021 21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K.C., Fang Y., Cao H., Chen H., Hu T., Chen Y., et al. Behavioral intention to receive a COVID-19 vaccination among Chinese factory workers: cross-sectional online survey. J Med Internet Res. 2021;23(3) doi: 10.2196/24673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/BMJ.N71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGowan J., Sampson M., Salzwedel D., Cogo E., Foerster V., Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/J.JCLINEPI.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app forsystematic reviews. Syst Rev. 2016;5:1–10. doi: 10.1186/S13643-016-0384-4. 2016 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WebPlotDigitizer - extract data from plots, images, and maps. https://automeris.io/WebPlotDigitizer/citation.html n.d.
- 20.Modesti P.A., Reboldi G., Cappuccio F.P., Agyemang C., Remuzzi G., Rapi S., et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/JOURNAL.PONE.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker T.H., Migliavaca C.B., Stein C., Colpani V., Falavigna M., Aromataris E., et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21:1–9. doi: 10.1186/S12874-021-01381-Z/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter J.P., Saratzis A., Sutton A.J., Boucher R.H., Sayers R.D., Bown M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897–903. doi: 10.1016/J.JCLINEPI.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Ticona J.P.A., Nery N., Victoriano R., Fofana M.O., Ribeiro G.S., Giorgi E., et al. Willingness to get the COVID-19 vaccine among residents of slum settlements. Vaccines. 2021;9:951. doi: 10.3390/VACCINES9090951. 2021;9:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinelli-Arzubiaga D., Marquez-Bravo A.W., Ortega I.G.A., Rodriguez-Alarcón J.F., Arias-Chavez D., Vilela-Estrada M.A., et al. Acceptance of COVID-19 vaccination among pregnant Peruvian women: attitudes and associated factors. Bol Malariol Salud Ambiental. 2021;61:45–52. doi: 10.52808/BMSA.7E5.61E2.005. [DOI] [Google Scholar]
- 25.Caycho-Rodríguez T., Carbajal-León C., Vivanco-Vidal A., Saroli-Araníbar D. Intention to vaccinate against COVID-19 in Peruvian older adults. Rev Esp Geriatr Gerontol. 2021;56:245–246. doi: 10.1016/J.REGG.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gramacho W.G., Turgeon M. When politics collides with public health: COVID-19 vaccine country of origin and vaccination acceptance in Brazil. Vaccine. 2021;39:2608–2612. doi: 10.1016/J.VACCINE.2021.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bono S.A., Villela EF. de M., Siau C.S., Chen W.S., Pengpid S., Hasan M.T., et al. Factors affecting COVID-19 vaccine acceptance: an international survey among low- and middle-income countries. Vaccines. 2021;9:515. doi: 10.3390/VACCINES9050515. Page 515 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skjefte M., Ngirbabul M., Akeju O., Escudero D., Hernandez-Diaz S., Wyszynski D.F., et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36:197–211. doi: 10.1007/S10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caycho-Rodríguez T., Tomás J.M., Carbajal-León C., Vilca L.W., Reyes-Bossio M., Intimayta-Escalante C., et al. Sociodemographic and psychological predictors of intention to receive a COVID-19 vaccine in elderly Peruvians. Trends in Psychol. 2021:1–18. doi: 10.1007/S43076-021-00099-7/TABLES/2. [DOI] [Google Scholar]
- 30.López-Cepero A., Cameron S., Negrón L.E., Colón-López V., Colón-Ramos U., Mattei J., et al. Uncertainty and unwillingness to receive a COVID-19 vaccine in adults residing in Puerto Rico: assessment of perceptions, attitudes, and behaviors. Hum Vaccines Immunother. 2021;17:3441–3449. doi: 10.1080/21645515.2021.1938921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melin K., Zhang C., Zapata J.P., Rivera Y.M., Fernandez K., Shacham E., et al. Factors associated with intention to receive vaccination against COVID-19 in Puerto Rico: an online survey of adults. Int J Environ Res Publ Health. 2021;18 doi: 10.3390/IJERPH18157743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vignier N., Brureau K., Granier S., Breton J., Michaud C., Gaillet M., et al. Attitudes towards the COVID-19 vaccine and willingness to get vaccinated among healthcare workers in French Guiana: the influence of geographical origin. Vaccines. 2021;9 doi: 10.3390/VACCINES9060682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stojanovic J., Boucher V.G., Gagne M., Gupta S., Joyal-Desmarais K., Paduano S., et al. Global trends and correlates of COVID-19 vaccination hesitancy: findings from the iCARE study. Vaccines. 2021;9:661. doi: 10.3390/VACCINES9060661. 2021;9:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaramillo-Monge J., Obimpeh M., Vega B., Acurio D., Boven A., Verhoeven V., et al. COVID-19 vaccine acceptance in azuay province, Ecuador: a cross-sectional online survey. Vaccines. 2021;9:678. doi: 10.3390/VACCINES9060678. Page 678 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazarus J.v., Wyka K., Rauh L., Rabin K., Ratzan S., Gostin L.O., et al. Hesitant or not? The association of age, gender, and education with potential acceptance of a COVID-19 vaccine: a country-level. Analysis. 2021;25:799–807. doi: 10.1080/10810730.2020.1868630. [DOI] [PubMed] [Google Scholar]
- 36.Villarreal-Garza C., Vaca-Cartagena B.F., Becerril-Gaitan A., Ferrigno A.S., Mesa-Chavez F., Platas A., et al. Attitudes and factors associated with COVID-19 vaccine hesitancy among patients with breast cancer. JAMA Oncol. 2021;7:1242–1244. doi: 10.1001/JAMAONCOL.2021.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castañeda-Vasquez D.E., Ruiz-Padilla J.P., Botello-Hernandez E. Vaccine hesitancy against SARS-CoV-2 in health personnel of northeastern Mexico and its determinants. J Occup Environ Med. 2021;63:633–637. doi: 10.1097/JOM.0000000000002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerda A.A., García L.Y. Hesitation and refusal factors in individuals' decision-making processes regarding a coronavirus disease 2019 vaccination. Front Public Health. 2021;9:229. doi: 10.3389/FPUBH.2021.626852/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvis-Guzman N., Alvis-Zakzuk J., Paz-Wilches J., Fernandez-Mercado J.C., de la Hoz-Restrepo F. Disposición a recibir la vacuna contra COVID-19 en población de 80 y más años en Colombia 2021. Vacunas. 2021 doi: 10.1016/J.VACUN.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9:160. doi: 10.3390/VACCINES9020160. 2021;9:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tracking covid-19 excess deaths across countries | the Economist. https://www.economist.com/graphic-detail/coronavirus-excess-deaths-tracker n.d.
- 42.Santirocchi A., Spataro P., Costanzi M., Doricchi F., Rossi-Arnaud C., Cestari V. Predictors of the intention to Be vaccinated against COVID-19 in a sample of Italian respondents at the start of the immunization campaign. J Personalized Med. 2022;12 doi: 10.3390/JPM12010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caserotti M., Girardi P., Rubaltelli E., Tasso A., Lotto L., Gavaruzzi T. Associations of COVID-19 risk perception with vaccine hesitancy over time for Italian residents. Soc Sci Med. 2021;272 doi: 10.1016/J.SOCSCIMED.2021.113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Detoc M., Bruel S., Frappe P., Tardy B., Botelho-Nevers E., Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;38:7002–7006. doi: 10.1016/J.VACCINE.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guillon M., Kergall P. Factors associated with COVID-19 vaccination intentions and attitudes in France. Publ Health. 2021;198:200–207. doi: 10.1016/J.PUHE.2021.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giannouchos T.v., Steletou E., Saridi M., Souliotis K. Mandatory vaccination support and intentions to get vaccinated for COVID-19: results from a nationally representative general population survey in October 2020 in Greece. J Eval Clin Pract. 2021;27:996–1003. doi: 10.1111/JEP.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sypsa V., Roussos S., Engeli V., Paraskevis D., Tsiodras S., Hatzakis A. Trends in COVID-19 vaccination intent, determinants and reasons for vaccine hesitancy: results from repeated cross-sectional surveys in the adult general population of Greece during november 2020-June 2021. Vaccines. 2022;10:470. doi: 10.3390/VACCINES10030470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandstetter S., Böhmer M.M., Pawellek M., Seelbach-Göbel B., Melter M., Kabesch M., et al. Parents' intention to get vaccinated and to have their child vaccinated against COVID-19: cross-sectional analyses using data from the KUNO-Kids health study. Eur J Pediatr. 2021;180:3405–3410. doi: 10.1007/S00431-021-04094-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graeber D., Schmidt-Petri C., Schröder C. Attitudes on voluntary and mandatory vaccination against COVID-19: evidence from Germany. PLoS One. 2021;16 doi: 10.1371/JOURNAL.PONE.0248372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherman S.M., Sim J., Cutts M., Dasch H., Amlôt R., Rubin G.J., et al. COVID-19 vaccination acceptability in the UK at the start of the vaccination programme: a nationally representative cross-sectional survey (CoVAccS - wave 2) Publ Health. 2022;202:1–9. doi: 10.1016/J.PUHE.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherman S.M., Smith L.E., Sim J., Amlôt R., Cutts M., Dasch H., et al. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum Vaccines Immunother. 2021;17:1612–1621. doi: 10.1080/21645515.2020.1846397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin Y., Hu Z., Zhao Q., Alias H., Danaee M., Wong L.P. Understanding COVID-19 vaccine demand and hesitancy: a nationwide online survey in China. PLoS Neglected Trop Dis. 2020;14 doi: 10.1371/JOURNAL.PNTD.0008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L., Wang J., Nicholas S., Maitland E., Leng A., Liu R. The intention to receive the COVID-19 vaccine in China: insights from protection motivation theory. Vaccines. 2021;9 doi: 10.3390/VACCINES9050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banik R., Islam M.S., Pranta M.U.R., Rahman Q.M., Rahman M., Pardhan S., et al. Understanding the determinants of COVID-19 vaccination intention and willingness to pay: findings from a population-based survey in Bangladesh. BMC Infect Dis. 2021:21. doi: 10.1186/S12879-021-06406-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kabir R., Mahmud I., Chowdhury M.T.H., Vinnakota D., Jahan S.S., Siddika N., et al. COVID-19 vaccination intent and willingness to pay in Bangladesh: a cross-sectional study. Vaccines. 2021;9 doi: 10.3390/VACCINES9050416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kollamparambil U., Oyenubi A., Nwosu C. COVID19 vaccine intentions in South Africa: health communication strategy to address vaccine hesitancy. BMC Publ Health. 2021;21:1–12. doi: 10.1186/S12889-021-12196-4/TABLES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. 2021. [DOI] [PMC free article] [PubMed]
- 58.Débora Acosta L. Capacidad de respuesta frente a la pandemia de COVID-19 en América Latina y el Caribe n.d. 10.26633/RPSP.2020.109. [DOI] [PMC free article] [PubMed]
- 59.COVID-19 in Latin America: where we stand and what is to come. https://www.giga-hamburg.de/en/publications/28577360-covid-19-latin-america-where-we-stand-what-is-to-come/ n.d.
- 60.Covid-19: Cómo llegó Sudamérica a liderar la carrera mundial de vacunación | Sociedad | EL PAÍS. https://elpais.com/sociedad/2022-01-07/como-llego-sudamerica-a-liderar-la-carrera-mundial-de-vacunacion.html?ssm=TW_CC n.d.
- 61.Sarasty O., Carpio C.E., Hudson D., Guerrero-Ochoa P.A., Borja I. The demand for a COVID-19 vaccine in Ecuador. Vaccine. 2020;38:8090. doi: 10.1016/J.VACCINE.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tahir A.I., Ramadhan D.S., Taha A.A., Abdullah R.Y., Karim S.K., Ahmed A.K., et al. Public fear of COVID-19 vaccines in Iraqi Kurdistan region: a cross-sectional study. Middle East Curr Psychiatr. 2021;28:1–8. doi: 10.1186/S43045-021-00126-4/FIGURES/3. [DOI] [Google Scholar]
- 63.Rief W. Fear of adverse effects and COVID-19 vaccine hesitancy: recommendations of the treatment expectation expert group. JAMA Health Forum. 2021;2 doi: 10.1001/JAMAHEALTHFORUM.2021.0804. e210804. [DOI] [PubMed] [Google Scholar]
- 64.Mayta-Tristán P., Aparco J.P. [Use of experimental vaccine outside of clinical trial: the “Vacunagate” case] Rev Peru Med Exp Salud Pública. 2021;38:203–205. doi: 10.17843/RPMESP.2021.382.8694. [DOI] [PubMed] [Google Scholar]
- 65.Kenyon G. Vacuna-gate escalates in Peru. Lancet Infect Dis. 2021;21:463. doi: 10.1016/S1473-3099(21)00157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muric G., Wu Y., Ferrara E. COVID-19 vaccine hesitancy on social media: building a public twitter data set of antivaccine content, vaccine misinformation, and conspiracies. JMIR Public Health Surveill. 2021;7 doi: 10.2196/30642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson S.L., Wiysonge C. Social media and vaccine hesitancy. BMJ Global Health. 2020;5 doi: 10.1136/BMJGH-2020-004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuehn B.M. Vaccine passports help boost lagging vaccination rates. JAMA. 2022;327:209. doi: 10.1001/JAMA.2021.23676. 209. [DOI] [PubMed] [Google Scholar]
- 69.Halperin D.T., Hearst N., Hodgins S., Bailey R.C., Klausner J.D., Jackson H., et al. Revisiting COVID-19 policies: 10 evidence-based recommendations for where to go from here. BMC Publ Health. 2021;21:1–12. doi: 10.1186/S12889-021-12082-Z. 2021 21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang S.J., Jung S.I. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020;52:154–164. doi: 10.3947/IC.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Angelo A.T., Alemayehu D.S., Dachew A.M. Health care workers intention to accept COVID-19 vaccine and associated factors in southwestern Ethiopia, 2021. PLoS One. 2021;16 doi: 10.1371/JOURNAL.PONE.0257109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iguacel I., Luna Maldonado A., Luna Ruiz-Cabello A., Samatán E., Alarcón J., Ángeles Orte M., et al. Attitudes of healthcare professionals and general population toward vaccines and the intention to Be vaccinated against COVID-19 in Spain. Front Public Health. 2021;9:1446. doi: 10.3389/FPUBH.2021.739003/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dzieciolowska S., Hamel D., Gadio S., Dionne M., Gagnon D., Robitaille L., et al. Covid-19 vaccine acceptance, hesitancy, and refusal among Canadian healthcare workers: a multicenter survey. Am J Infect Control. 2021;49:1152–1157. doi: 10.1016/J.AJIC.2021.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abu-Farha R.K., Alzoubi K.H., Khabour O.F. <p>Public willingness to participate in COVID-19 vaccine clinical trials: a study from Jordan</p>. Patient Prefer Adherence. 2020;14:2451–2458. doi: 10.2147/PPA.S284385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malik A.A., McFadden S.A.M., Elharake J., Omer S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26 doi: 10.1016/J.ECLINM.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruiz J.B., Bell R.A. Predictors of intention to vaccinate against COVID-19: results of a nationwide survey. Vaccine. 2021;39:1080–1086. doi: 10.1016/J.VACCINE.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sallam M., Dababseh D., Eid H., Al-Mahzoum K., Al-Haidar A., Taim D., et al. High rates of COVID-19 vaccine hesitancy and its association with conspiracy beliefs: a study in Jordan and Kuwait among other arab countries. Vaccines. 2021;9:42. doi: 10.3390/VACCINES9010042. Page 42 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong L.P., Alias H., Wong P.F., Lee H.Y., AbuBakar S. vol. 16. 2020. p. 2204. (The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay). 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ceulemans M., Foulon V., Panchaud A., Winterfeld U., Pomar L., Lambelet V., et al. Vaccine willingness and impact of the COVID-19 pandemic on women's perinatal experiences and practices-A multinational, cross-sectional study covering the first wave of the pandemic. Int J Environ Res Publ Health. 2021;18 doi: 10.3390/IJERPH18073367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mappa I., Luviso M., Distefano F.A., Carbone L., Maruotti G.M., Rizzo G. Women perception of SARS-CoV-2 vaccination during pregnancy and subsequent maternal anxiety: a prospective observational study. J Matern Fetal Neonatal Med. 2021 doi: 10.1080/14767058.2021.1910672. [DOI] [PubMed] [Google Scholar]
- 81.Gencer H., Özkan S., Vardar O., Serçekuş P. The effects of the COVID 19 pandemic on vaccine decisions in pregnant women. Women Birth. 2021 doi: 10.1016/J.WOMBI.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.COVID-19 map - Johns Hopkins coronavirus resource center. https://coronavirus.jhu.edu/map.html n.d.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.