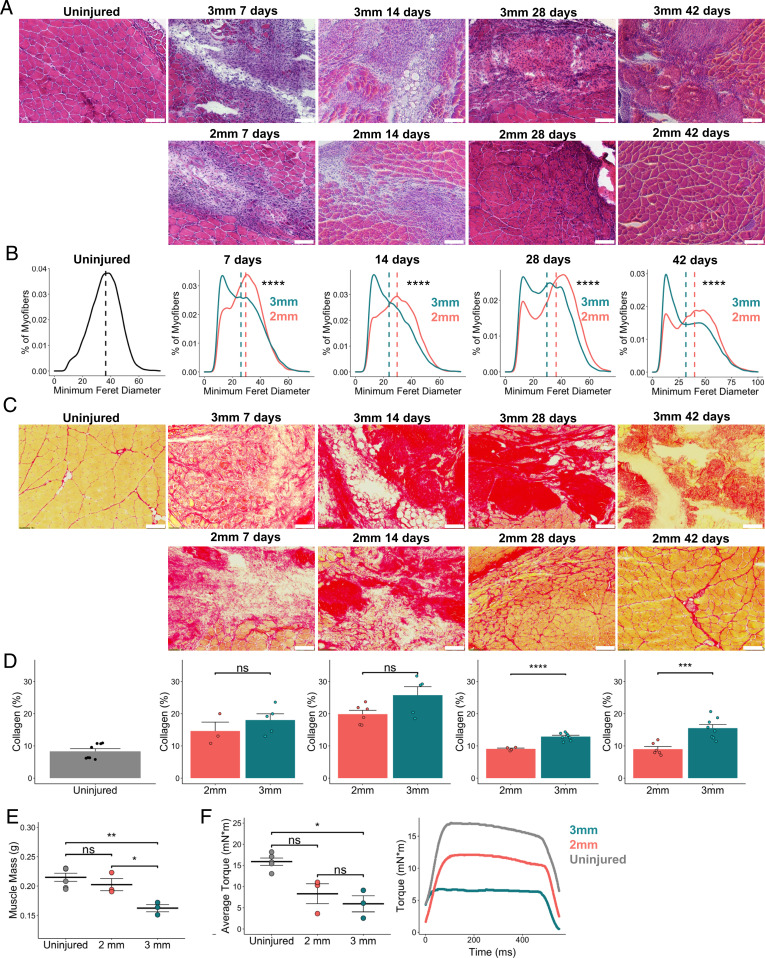
Fig. 1.
Degenerative VML defects exhibit increased fibrosis and reduced myofiber diameters. (A) Representative H&E stains of uninjured quadriceps along with 2-mm and 3-mm defects harvested 7, 14, 28, and 42 dpi. (Scale bars, 200 μm.) Single windows of the defect area are shown for detail. Representative full-section images are shown in SI Appendix, Fig. S1. (B) Portion of myofibers stratified by minimum Feret diameter shows smaller fiber size distribution following 3-mm defect. ****P < 0.0001 by two-sample, two-sided Kolmogorov–Smirnov test for equal distributions. Feret diameters were calculated for 5 to 7 full-section images each from a distinct defect, per group, and pooled for comparison of distributions. Dashed lines indicate mean minimum Feret diameters. (C) Representative PSR stains of 2-mm and 3-mm defects throughout the time course. (D) Quantification of collagen content showing a return to preinjury levels following 2-mm defects by 28 dpi but a significant increase and persistence following 3-mm defects. (Scale bars, 200 μm.) Bars show mean ± SEM; ns denotes not significant (P > 0.05). ****P < 0.0001, ***P < 0.001 by two-sided t test. n = 3 to 7 tissues per group. Cohen’s d = 0.7566, 1.2678, 2.2536, and 2.4264 for 7, 14, 28, and 42 dpi, respectively. Quantifications were performed on full section stitched images (representative images shown in SI Appendix, Fig. S1). Single windows of the defect area are shown for detail. (E) Muscle mass is significantly reduced among limbs that received 3-mm defects at 28 dpi compared to uninjured limbs and those that received 2-mm defects. **P < 0.01, *P < 0.05, ns denotes P > 0.05 by one-way ANOVA and posthoc analysis. n = 3 to 5 tissues per group. Bars show mean ± SEM. (F) Average isometric torque (Left) and representative force curves (Right) of uninjured quadriceps or quadriceps 28 d following 2-mm or 3-mm injuries. *P < 0.05, ns denotes P > 0.05 by one-way ANOVA and posthoc analysis. n = 3 to 5 tissues per group. Bars show mean ± SEM.